As generally known, the size of Pt NPs is one of the most important factors affecting the performance of a catalyst. With the decreasing of particle size, a large fraction of active Pt atoms could be exposed to the surfaces; thus, ultrafine Pt NPs often exhibit highly enhanced catalytic activities. At the same time, downsizing Pt NPs is another way to reduce the Pt loading and cost of catalysts. However, when the size of the Pt NPs is too small, the high surface energy of the NPs may cause severe aggregation, thereby inducing a serious decline in their performance during catalytic operation and impeding their use in industrial processes. Generally, an effective strategy to solve these deactivation problems involves uniform loading of the Pt NP catalysts onto a carrier with a large surface area and good electrical conductivity.
Based on the large surface area and high electrical conductivity of graphene, the research group at Institute of Solid State Physics, Hefei Institutes of Physical Science, prepared ultrafine platinum/reduced graphene oxide (Pt/rGO) nanocomposites (the size of Pt NPs: 1.8 (±0.6) nm), which was published in journal of ACS Appl. Mater. Interfaces with title Highly Dispersed Ultrafine Pt Nanoparticles on Reduced Graphene Oxide Nanosheets: In Situ Sacrificial Template Synthesis and Superior Electrocatalytic Performance for Methanol Oxidation.
Typically, LAL-generated MnOx colloids with high reactivity can be used as agents to reduce GO and PtCl62- , and MnOx NPs can be easily deposited onto graphene nanosheets and further used as in situ sacrificial templates to ensure the uniform distribution of reduced Pt NPs on the surface of rGO nanosheets.
To identify the potential utility in catalytic applications, the electrocatalytic activity of the as-prepared Pt/rGO NCs was tested for methanol oxidation. Results revealed that the specific activities of Pt/rGO catalysts are much higher than those of the commercial Pt/C catalysts in acidic and alkaline media.
Moreover, the superior electrocatalytic activity, excellent oxidation ability and corrosion resistance are also exhibited in the prepared Pt/rGO catalysts. The good performance of as-prepared Pt/rGO catalysts in methanol oxidation can be reasonably understood by considering the following facts.
Firstly, compared with other carbon support materials, graphene possesses a larger surface area that can efficiently prevent the Pt NPs from coalescing and thus lead to the formation of highly dispersed, high-density, and ultrafine Pt NPs.
Then, these well-dispersed and ultrafine Pt NPs are endowed with more electrochemically active sites, active facets, and an active surface area.
At last, the excellent stability of graphene can retain the structural integrity of the Pt/rGO catalysts under harsh electrochemical conditions. In addition, graphene has excellent conductivity and can cause rapid transport of electron or charge carriers.
This study was sponsored by the National Basic Research Program of China, the National Natural Science Foundation of China and CAS Key Technology Talent Program.
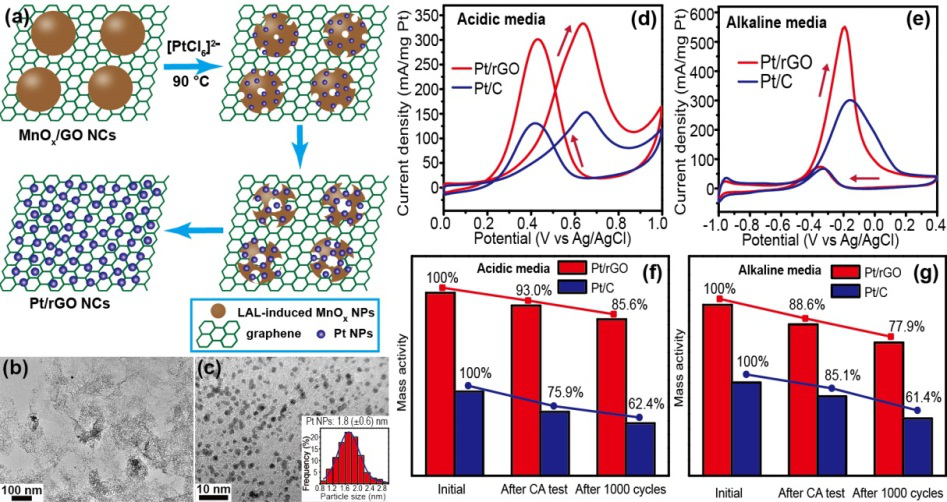
Pt/rGO catalysts: (a) Schematic Illustration for the Formation of Pt/rGO NCs; (b-c) TEM images of Pt/rGO NCs and particle size distribution histogram of Pt NPs; (d-e) CV curves of GC electrodes modified with prepared Pt/rGO and commercial Pt/C catalysts measured in acidic and alkaline media; (f-g) the catalysis stability of Pt/rGO and commercial Pt/C catalysts measured in acidic and alkaline media.(Image by WU Shouliang)
Contact:
LIANG Changhao
Institute of Solid State Physics (http://english.issp.ac.cn/)
Hefei 230031, China.
Tel: +86 (0)551 65594860
E-mail: chliang@issp.ac.cn
 Tel: +86-551-65591206
Tel: +86-551-65591206
 Fax: +86-551-65591270
Fax: +86-551-65591270
 Emai: zhous@hfcas.ac.cn
Emai: zhous@hfcas.ac.cn
 350 Shushanhu Road
350 Shushanhu Road