The group of Prof. ZHANG Weijun at Anhui Institute of Optics and Fine Mechanics (AIOFM), Hefei Institute of Physical Science, in cooperation with National Synchrotron Radiation Laboratory (NSRL), University of Science and Technology of China, has made a new progress in the pyrolysis of n-butane by using synchrotron threshold photoelectron photoion coincidence spectroscopy (TPEPICO) as well as revealed its detailed mechanisms.
The method of TPEPICO collects both electrons and ions simultaneously in photoionization, and which is powerful to provide multiplex information in the field of chemical dynamics.
Dr. TANG Xiaofeng, one staff of the group, has been focusing on the technical development of TPEPICO and its applications for several years. Just very recently, TANG and his co-workers investigated the pyrolysis n-butane, the prototype of hydrocarbons and an essential model fuel in combustion, by utilizing the TPEPICO setup and synchrotron radiation.
The thermal decomposition reactions of n-butane are important initiation steps in the high-temperature oxidation and have strong influence on the ignition time.
In their study, the team thermally decomposed N-butane in a home-made flash pyrolysis micro-reactor and determined primary products with TPEPICO time-of-flight mass spectra and mass-selected threshold photoelectron spectra (TPES).
In addition, they observed vibrational structures in the TPES of the CH3, C2H5 and C3H6 products, moreover, whose ionization energies were measured and compared satisfactorily to literature data.
Besides, using high-level quantum chemistry the team calculated potential energy surface involved in the decomposition and recognized several transition states. Then based on the experimental data and the theoretical results, they revealed the detailed mechanisms of the pyrolysis of n-butane.
Their work, entitled Pyrolysis of n-butane investigated using synchrotron threshold photoelectron photoion coincidence spectroscopy are detailed in RSC Advances (2017, 7, 28746-28753).
The work was supported by the National Key Research and Development Program of China, the National Natural Science Foundation of China and the Natural Science Foundation of Anhui Province.
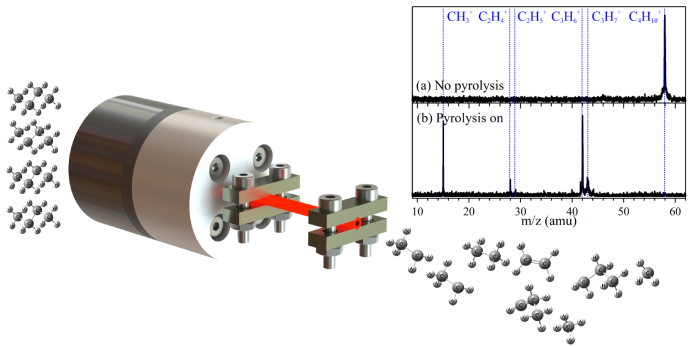
Fig. 1 Schematic diagram of the flash pyrolysis setup and the time-of-flight mass spectra of n-butane with the pyrolysis power on and off. (Imaged by TANG Xiaofeng)
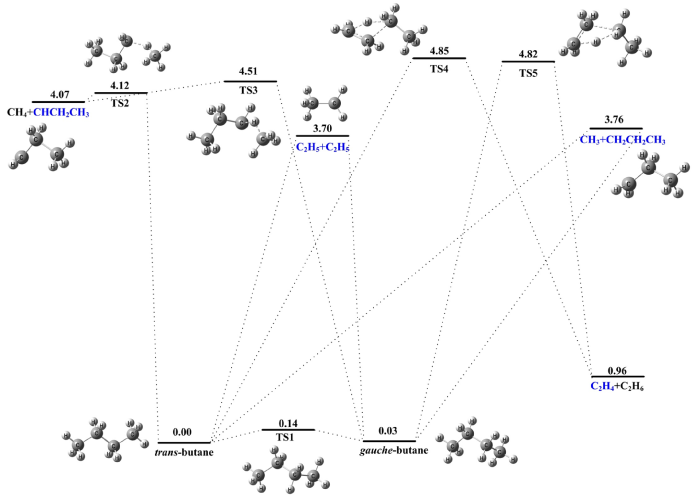
Fig. 2. Potential energy surface and relative energy of the primary products involved in the pyrolysis of n-butane (Imaged by LIN Xiaoxiao)
Contact:
TANG Xiaofeng
Anhui Institute of Optics and Fine Mechanics (http://www.aiofm.cas.cn/)
Hefei 230031, Anhui, China
Tel:+86-551-65590357
E-mail:tangxf@aiofm.ac.cn
 Tel: +86-551-65591206
Tel: +86-551-65591206
 Fax: +86-551-65591270
Fax: +86-551-65591270
 Emai: zhous@hfcas.ac.cn
Emai: zhous@hfcas.ac.cn
 350 Shushanhu Road
350 Shushanhu Road