Scientists from the Institute of Solid State Physics, Hefei Institutes of Physical Science developed the alfalfa-derived nitrogen (N)-doped porous carbon (NPC) fabricated by a pyrolysis method and it was electrocatalytically active for the NRR.
Electrosynthesis of ammonia (NH3) from the nitrogen (N2) reduction reaction (NRR) at ambient conditions has been widely regarded as a “green NH3 synthesis” technology to replace traditional energy- and capital-intensive Haber-Bosch process.
Until recently, metal-free N-doped porous graphitic carbon (NPGC) materialsmetal-free N-doped porous graphitic carbon (NPGC) materials as the electrocatalysts have demonstrated good NRR activities, promising for NH3 synthesis.
However, two main issues using the NPGC electrocatalysts for NRR still raise concern: one is the NRR active mechanism and another is if the doped N in NPGC catalyst will break away from the catalyst surface to contribute the NH3 formation during NRR.
It is well known that natural biomass resources provide sufficient raw materials for the future development and application of carbon materials because of their rich carbon content.
The results demonstrate that the doped pyridinic-N in NPC-500 catalyst during NRR could easily break away from its surface to form N vacancies in carbon matrix as the catalytic active sites for the NRR.
As a proof of concept experiment, they further utilized the NPC-500 assembled Zn-air battery to drive the NPC-500 constructed two-electrode NRR cell, giving a high NH3 yield rate and Faradaic efficiency.
The findings in this work demonstrates the feasibility of utilizing biomass-derived carbon electrocatalyst for energy-integrated electrosynthesis of NH3 from the N2 reduction reaction at ambient conditions.
Link to the paper: Ambient Electrosynthesis of Ammonia on a Biomass-Derived Nitrogen-Doped Porous Carbon Electrocatalyst: Contribution of Pyridinic Nitrogen
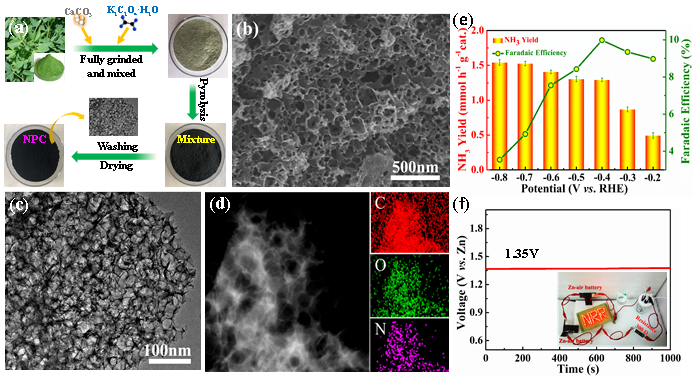
Figure. (a) A schematic illustration of the fabrication process of alfalfa-derived NPC sample. (b) Surface SEM image of the NPC-500. (c) TEM image of the NPC-500. (d) HAADF-STEM image and corresponding elemental mapping images of the NPC-500. (e) NH3 yield rate and Faradaic efficiency of the NPC-500 at different potentials in N2-saturated 0.005 M H2SO4 solution. (f) Voltage-time curve of the NPC-500 assembled Zn-air battery.(Image by ZHAO Cuijiao)
Contact:
ZHOU Shu
Hefei Institutes of Physical Science (http://english.hf.cas.cn/)
Email: zhous@hfcas.ac.cn
 Tel: +86-551-65591206
Tel: +86-551-65591206
 Fax: +86-551-65591270
Fax: +86-551-65591270
 Emai: zhous@hfcas.ac.cn
Emai: zhous@hfcas.ac.cn
 350 Shushanhu Road
350 Shushanhu Road