The research group led by Prof. ZHANG Weijun at Anhui Institute of Optics and Fine Mechanics, Hefei Institutes of Physical Science has made new progress on the vacuum ultraviolet photodynamics of the methyl peroxy radical (CH3O2).
Peroxy radicals (RO2) are important reaction intermediates in the atmosphere. Their reactions can lead to the formation of secondary pollutants such as ozone and fine particles, taking a large influence on the atmospheric environment and human health. The structure, thermochemistry and kinetics of RO2 are of considerable importance in order to fully understand their chemistry in the atmosphere.
TANG Xiaofeng, one staff of the group, worked in international collaboration with the scientists of Synchrotron SOLEIL, France, to investigate the vacuum ultraviolet (VUV) photoionization of the methyl peroxy radical, CH3O2, the most abundant peroxy radical in the atmosphere, by using the state-of-the-art method of double imaging photoelectron photoion coincidence (i2PEPICO). In addition, the unimolecular dissociation of internal energy selected CH3O2+ cations was also been studied in detail.
In their work, a microwave discharge flow tube was employed as reactor to produce CH3O2 via the reaction of methyl radicals (CH3) with oxygen gas. The high resolution slow photoelectron spectrum (SPES) of CH3O2 was obtained exhibiting two broad bands superimposed with a complex vibrational structure.
The first band of the SPES was attributed to the X3A" and a1A’ overlapped electronic states of CH3O2+ and the second was assigned to the b1A’ electronic state with the help of theoretical calculations.
The adiabatic ionization energy (AIE) of CH3O2 was derived as 10.215 ± 0.015 eV, in good agreement with high-accuracy theoretical data in the literature. The vertical ionization energy of the b1A' electronic state was measured to be 11.5 eV and this state fully dissociates into CH3+ and O2 fragments. The 0 K adiabatic appearance energy (AE0K) of the CH3+ fragment ion was determined to be 11.15 ± 0.02 eV.
This work was financially supported by the National Natural Science Foundation of China (Nos. 21773249, 91961123, 91544228) and the International Partnership Program of Chinese Academy of Sciences (No. 116134KYSB20170048).
Link to the paper: Vacuum Ultraviolet Photodynamics of the Methyl Peroxy Radical Studied by Double Imaging Photoelectron Photoion Coincidences
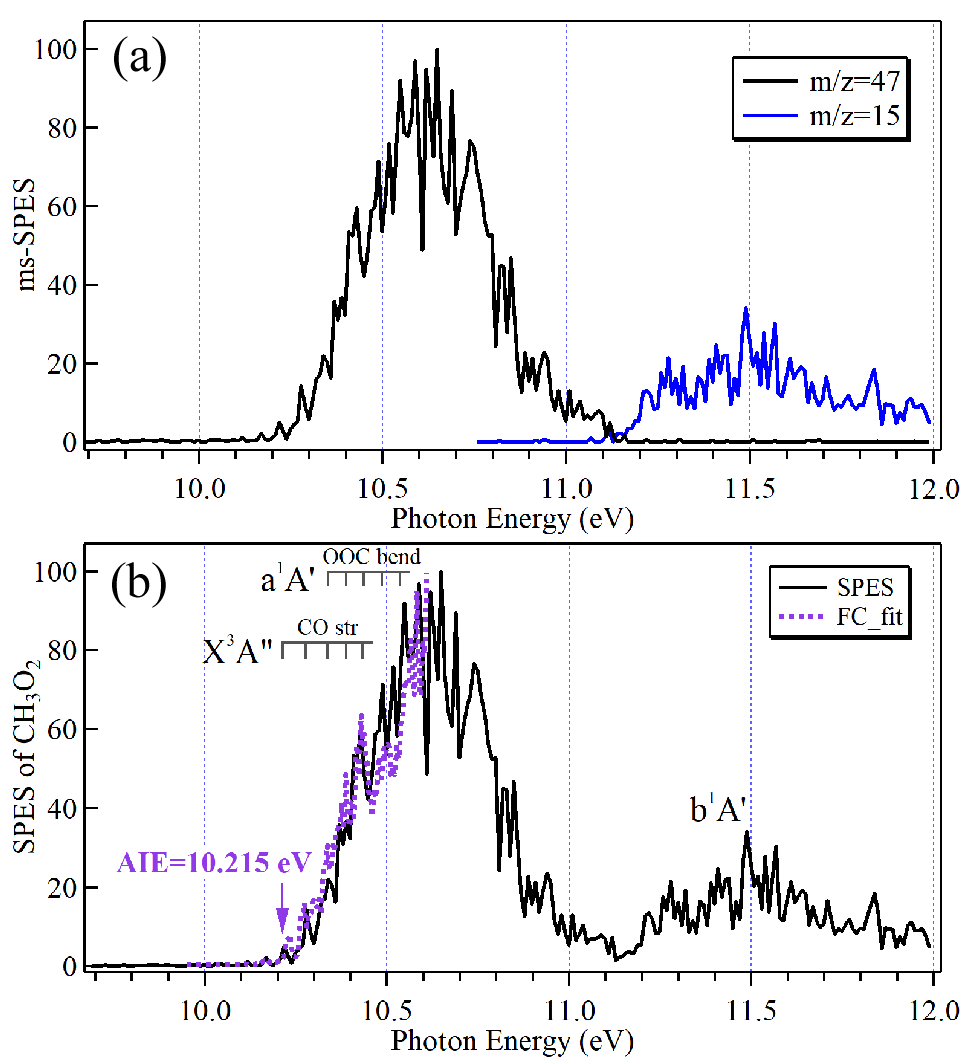
(a) Mass-selected slow photoelectron spectra (SPES) corresponding to m/z = 47 and 15 ions; (b) SPES of CH3O2 (black) and its partial Franck–Condon fitting (purple). (Image by LIN Xiaoxiao)
Contact:
ZHOU Shu
Hefei Institutes of Physical Science (http://english.hf.cas.cn/)
Email: zhous@hfcas.ac.cn
 Tel: +86-551-65591206
Tel: +86-551-65591206
 Fax: +86-551-65591270
Fax: +86-551-65591270
 Emai: zhous@hfcas.ac.cn
Emai: zhous@hfcas.ac.cn
 350 Shushanhu Road
350 Shushanhu Road