A Chinese research group explored the electrocatalytic interaction between the sensing interface and the target heavy metal, specifically As(III), by using Co single-atom catalyst (Co SAC) for the first time.
This work was done by HUANG Xingjiu and his team with Institute of Intelligent Machines, Hefei Institutes of Physical Science.
Although electrochemical technologies have been widely investigated for heavy metal ions (HMIs) detection, it is still a huge challenge to achieve highly sensitive and selective detection for HMIs. Moreover, many researchers in this field mainly focus on electrochemical performance, but less attention is paid to the corresponding detection mechanism. Actually, electrocatalytic reaction process takes the core point of electrochemical detection which lacks deep exploration especially from the perspective of electrocatalysis.
In this work, the team successfully characterized Co SAC and applied it to electrochemical detection of As(III). after their performance, they obtained an ultra-high sensitivity of 11.44 μA ppb-1 for As(III), which was much better than most of noble metallic catalysts.
And then, they did further work to explore the electrochemical behaviors of typical divalent HMIs and the results they got to confirm a superior selectivity for As(III).
In addition, The team also conduct another approach through X-ray absorption fine structure (XAFS) spectra and density function theory (DFT) calculation, and they found that H3AsO3 molecules were activated catalytically by Co-N2C2 active sites with the formation of Co-O hybridization bonds, causing lower energy barriers for the As-O dissociation steps in H3AsO3 reduction on Co SAC.
Moreover, reaction kinetics simulation done by the team also showed that the first electron transfer was the rate-limiting step of H3AsO3 reduction and much faster on Co SAC than that on Co nanoparticles material, then leading to a large amount deposition of As(0), which facilitated electrochemical response signals of As(III).
The team further reported that selectivity for As(III) was attributed to the formation of Co-O hybrid bonds as there was no specific interaction site between Co SAC and the bivalent HMIs without oxygen anion.
This work may expand application of SACs in electroanalysis field by adopting single-atom catalyst (SAC) as the sensing material in the electroanalysis of HMIs for the first time. And their investigations on the working mechanism of Co SAC provide atomic-level catalytic insights and important theoretical guidance for designing functional sensing interfaces from the perspective of electrocatalysis. Meanwhile, it also affords inspiration for catalytic treatment and removal of such pollutants in the water environment.
This work was supported financially by the National Natural Science Foundation of China (21735005, 21802145, U19A2015), the China Postdoctoral Innovation Talents Supporting Project (BX20180311), the China Postdoctoral Science Foundation (2019M652220), National Science Fund for Distinguished Young Scholars (21925204), Key Research Program of Frontier Sciences of the CAS (QYZDB-SSW-SLH017), Major Program of Development Foundation of Hefei Center for Physical Science and Technology (2017FXZY002), and USTC Research Funds of the Double First-Class Initiative (YD2340002002). C.H. Lin acknowledges the One Hundred Person Project of the Chinese Academy of Sciences, China, for financial support.
Link to the paper: Ultra-Sensitive and Selective Detection of Arsenic(III) via Electroanalysis over Cobalt Single-Atom Catalysts

Morphology and structure characterization of Co SAC. (Imaged by LI Peihua)
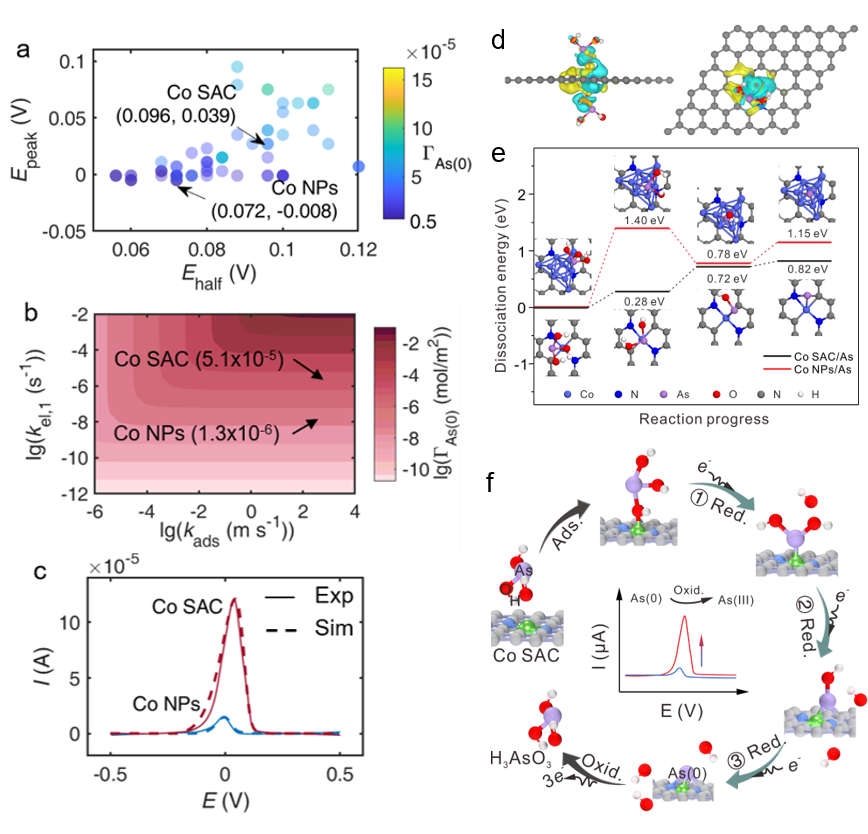
a)-c) Reaction kinetics simulation analysis. d) and e) DFT calculation. f) Mechanism diagram of enhanced electrochemical detection for As(III). (Imaged by LI Peihua)
Contact:
ZHOU Shu
Hefei Institutes of Physical Science (http://english.hf.cas.cn/)
Email: zhous@hfcas.ac.cn
 Tel: +86-551-65591206
Tel: +86-551-65591206
 Fax: +86-551-65591270
Fax: +86-551-65591270
 Emai: zhous@hfcas.ac.cn
Emai: zhous@hfcas.ac.cn
 350 Shushanhu Road
350 Shushanhu Road