Recently, scientists at Institute of Solid State Physics, Hefei Institutes of Physical Science developed a non-precious alloy catalyst with excellent catalytic activity, selectivity and stability for selective hydrogenation (SH) of cinnamaldehyde (CAL).
The researchers reported, for the first time, a solution-based metal ion impregnation approach was innovatively combined with pyrolysis to achieve the controllable synthesis of highly dispersed Fe-Co alloy nanoparticles (NPs) on N-doped graphitic carbon support.
They stated that the COL selectivity and CAL conversion efficiency were respectively promoted by the presence of Co and Fe, while the synergism of the alloyed Fe-Co was the key to concurrently achieve high COL selectivity and CAL conversion efficiency.
Another finding was the H2O played an important role in CAL conversion. For CAL conversation, the higher the solvent polarity, the higher the CAL conversion efficiency. For COL selectivity, it was categorically confirmed that the promotional effect of H2O solvent remarkably enhanced the hydrogenation on C=O and concurrently suppressed the hydrogenation on C=C.
COL has been an essential compound highly demanded by flavoring, perfume and pharmaceutical industries. But SH of CAL to COL is challenged by the unfavored thermodynamics that leads to low selectivity toward COL. And the use of organic solvent in COL production pollutes the environment and requires post-production separation.
To improve the selectivity of CAL hydrogenation, transition-metal/noble-metal (Pt is the most used) bimetallic catalysts is employed to create synergetic electronic effects that thermodynamically favoured the hydrogenation of C=O in CAL. However, the use of scarce and expensive noble-metals is undesirable.
This work was supported by the Natural Science Foundation of China (Grant No. 51871209 and 51902311) and the Postdoctoral Science Foundation of China (Grant No. 2019M652223).
Link to paper: Fe‐Co Alloyed Nanoparticles Catalysed Efficient Hydrogenation of Cinnamaldehyde to Cinnamyl Alcohol in Water
十日内
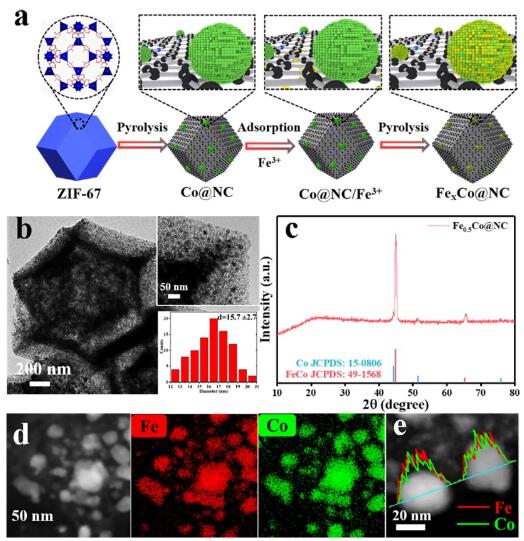
Figure 1. (a) Schematic illustration of synthetic procedure of FeXCo@NC; (b) TEM image (insets: high-magnification TEM image and Fe-Co NPs size distribution); (c) XRD pattern; (d-e) HAADF-STEM, corresponding elemental mapping images and line profile of Fe-Co NPs.(Image by LV Yang)
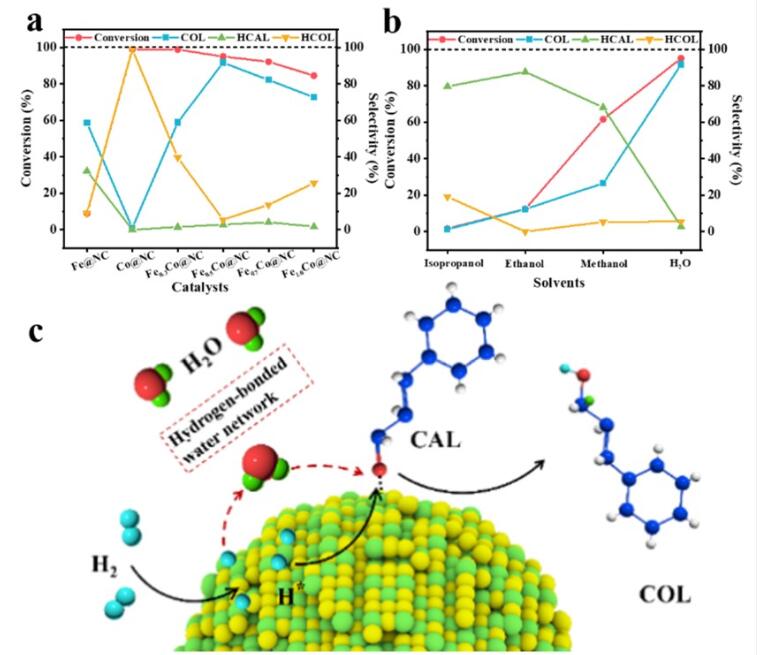
Figure 2. (a) Catalytic performance of Fe@NC, Co@NC and FeXCo@NC (80°C, 2MPa H2, 2h, H2O). (b) Effect of solvent (80°C, 2 MPa H2, 2h) on CAL conversion efficiency and COL selectivity of Fe0.5Co@NC catalysed SH of CAL to COL; (c) Schematic illustration of the hydrogenation of CAL over Fe-Co alloy catalyst in H2O. (Image by LV Yang)
Contact:
ZHOU Shu
Hefei Institutes of Physical Science (http://english.hf.cas.cn/)
Email: zhous@hfcas.ac.cn
 Tel: +86-551-65591206
Tel: +86-551-65591206
 Fax: +86-551-65591270
Fax: +86-551-65591270
 Emai: zhous@hfcas.ac.cn
Emai: zhous@hfcas.ac.cn
 350 Shushanhu Road
350 Shushanhu Road