Recently, a research team led by Prof. WANG Junfeng and Dr. ZHOU Shu, from the High Magnetic Field Laboratory, Hefei Institutes of Physical Science (HFIPS), successfully resolved the three-dimensional structure of voltage-gated Tim23 channel.
The team determined the structure in complex with a mitochondrial presequence peptide, using the method of solution-state NMR. “Tim23 channel structure was never reported before. This is the first time,” said ZHOU Shu, member of the team.
Most mitochondrial proteins are synthesized in the cytosol and transported into various mitochondrial subcompartments. Tim23 protein, the key component of the TIM23 complex forming a channel in the mitochondrial inner membrane, is believed to recognize and translocate precursor proteins into the mitochondrial matrix or to release them into the mitochondrial inner membrane.
Therefore, in recent years, lots of research have been conducted on the function of Tim23 channel, but structure of it was still unknown.
In this research, scientists found presequence binds to a coiled-coil motif in the mitochondrial intermembrane space via electrostatic forces. A short helix containing salt bridges served as a voltage sensor and aromatic rings at the membrane interface serve as a lock to operate channel gating, which was validated by electrophysiological analysis on the structure-based sequence variants.
The study offers mechanistic insight into how a voltage-sensitive channel transfers precursor protein into the mitochondrial matrix.
This study was supported by grants from the Ministry of Science and Technology of China and the National Natural Science Foundation of China, and funded by the Horizon 2020 program of the European Commission.
The NMR experiment was supported by the High Magnetic Field Laboratory of Chinese Academy of Sciences and the Biomolecular Magnetic Resonance Center at University of Frankfurt.
Link to the paper: Solution structure of the voltage-gated Tim23 channel in complex with a mitochondrial presequence peptide
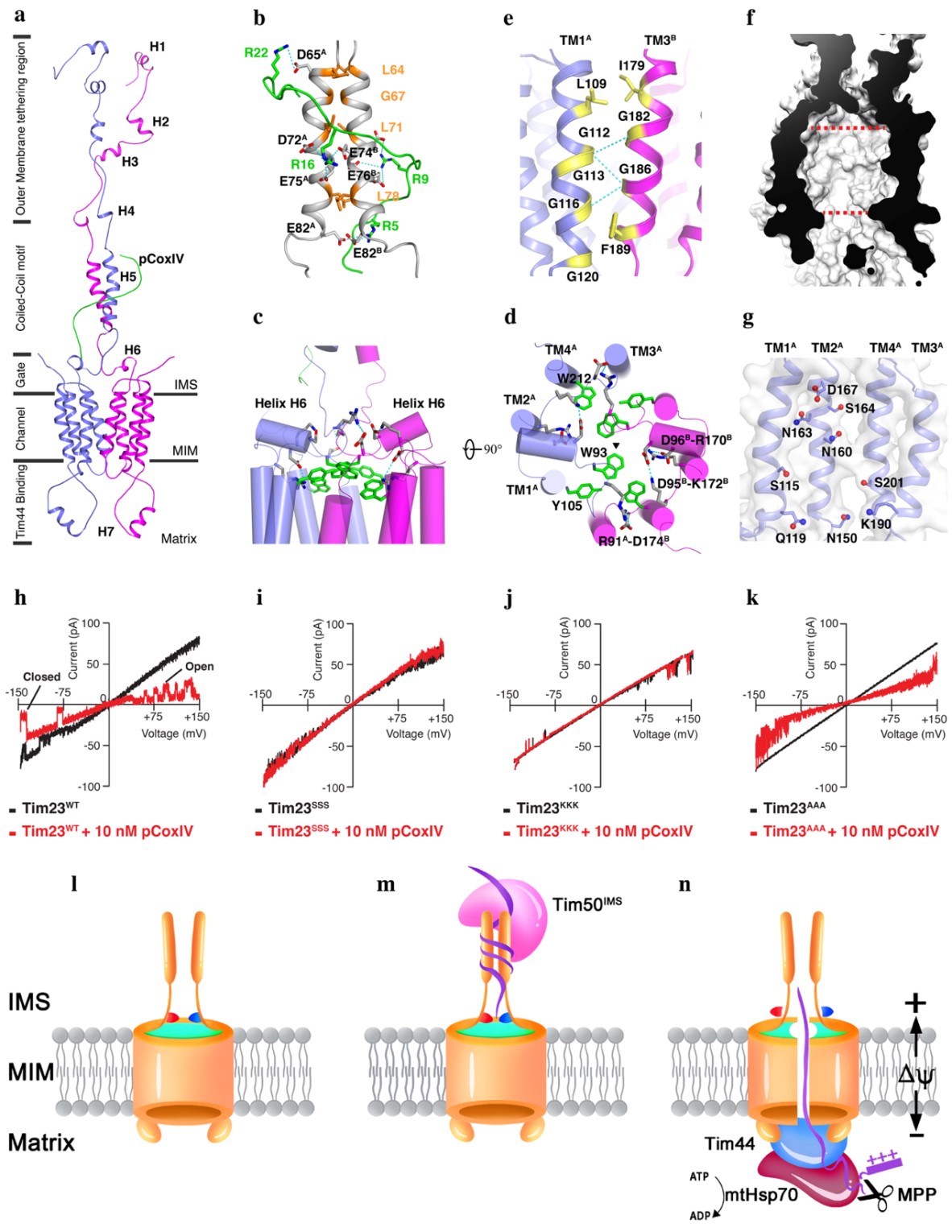
Cartoon representation of the Tim23-pCoxIV structure (a-g); electrophysiological analysis of the Tim23 wild type and Tim23 sequence variant channels in the absence or presence of pCoxIV (h-j); the proposed mechanism whereby the Tim23 channel transports precursor proteins into the mitochondrial matrix (l-n). (Image by ZHOU Shu)
Contact:
ZHAO Weiwei
Hefei Institutes of Physical Science (http://english.hf.cas.cn/)
Email: annyzhao@ipp.ac.cn
 Tel: +86-551-65591206
Tel: +86-551-65591206
 Fax: +86-551-65591270
Fax: +86-551-65591270
 Emai: zhous@hfcas.ac.cn
Emai: zhous@hfcas.ac.cn
 350 Shushanhu Road
350 Shushanhu Road