|
Recently, a new protein tyrosine phosphorylation quanlitative and quantitative analysis method was developed by double adjunct Prof. Changlin Tian of High Magnetic Field Laboratory, Chinese Academy of Sciences (CHMFL) and School of Life Sciences, University of Science and Technology of China (USTC) collaborating with Prof. Jiangyun Wang, Prof. Weiming Gong in Institute of Biophysics, Chinese Academy of Sciences, using 19F labeled unnatural amino acids and solid state NMR.
Tyrosine phosphorylation is a very important cellular post-translational modification, in regulating eukaryotic cell cycle, transcription activation and neuronal signal transduction. Disorders of tyrosine phosphorylation will leads to many human diseases, including cancer. In this study, 19F2-3,5-Tyrosine (Tyr-F2, an Tyrosine analogue) was site-specifically incorporated in several tyrosine kinases or their reactants through addition of Tyr-F2 in culture medium during protein production. Different 19F chemical shift values can be directly observed to distinguish protein tyrosine phosphorylation or dephosphorylation, while intensities of NMR peaks can provide quantative information of tyrosine phosphorylation.
Using the 19F solid state NMR methods, tyrosine phosphorylation levels of E. coli tyrosine kinases, Homo sapiens tyrosine kinase src (a vital enzyme in oncogenesis) were detected. And inhibition interaction of anti-cancer drug dasatinib against Src was also analyzed, which will provide a good reference and basis for further inhibitive drugs design or optimization against tyrosine kinases.
The 19F NMR experiments were conducted on the 400 MHz wide bore solid state NMR spectrometer in Public Experimentation Center of USTC and the 600 MHz wide bore solid state NMR spectrometer in High Magnetic Field Laboratory, Chinese Academy of Sciences.
This paper has been online reported in Angewandte Chemie International Edition on Feb. 28th, 2013, titled “A Genetically Encoded 19F NMR Probe for Tyrosine Phosphorylation” ( http://onlinelibrary.wiley.com/doi/10.1002/ange.201300463/abstract ). Two doctoral graduate students, Pan Shi and Jiasong Li in USTC, and one post-doctoral researcher, Dr. Fahui Li in Institute of Biophysics, CAS share the first-authors.
Magnetic Resonance has many special capabilities in protein structure determination and mechanism studies. Using the method of electron spin resonance, Prof. Changlin Tian has analyzed electron transportation pathway and mechanisms during reductive process of the type II mitocondria NADH dehydrogenase, an important membrane protein conducting red-ox process in respiration chain, in collaboration with Prof. Maojun Yang’s group in Tsinghua University. Related work has been published in Nature (Nature. 2012 Nov 15;491(7424):478-82) recently. Using the method of nuclear magnetic resonance, Prof. Changlin Tian and Dr. Fangming Wu in CHMFL has determined structure of outer membrane protein mmpS4 of Mycobacterium tuberculosis, and analyzed its virulence related siderophore properties, in collaboration with Prof. Michael Niederwas in University of Alabama, Birmingham, USA. The related work was reported in a top microbiology journal PLos Pathogens (PLOS Pathogens, 2013, Jan., 9(1):e1003120).
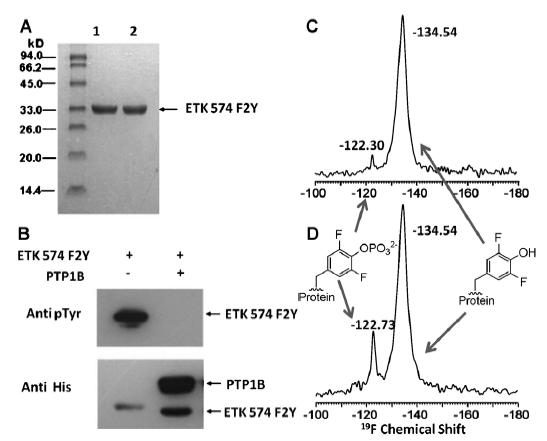
E. coli Tyrosine Phosphorylation Detection using 19F Nuclear Magnetic Resonance
|