F-g-C3N4 , a new type of polymer nanodots, which can detect Fe3+ and Cu2+ ions, is developed via hydrothermal treatment of bulk g-C3N4 by Prof. Xiangke Wang Team in Plasma Application Division, ASIPP, collaborating with Prof. Jinzhang Xu from HeFei University of Technology (HFUT).
Iron and copper are two important nutrient elements for human health, which rank as the second and third most essential trace metal elements in the human body. They also play significant roles in the nature environment. However, excessive intake or emission of iron or copper will lead to diseases and disasters. Therefore, it is particularly important for human health and monitoring of the environment to develop practical and efficient technologies for field analysis and rapid determination of iron and copper ions with high sensitivity and selectivity.
Scientists in ASIPP developed an extremely simple and green hydrothermal treatment of bulk g-C3N4 to form fluorescent g-C3N4 (F-g-C3N4) dots with blue emission and high quantum yield (QY), which were applied as a very effective fluorescent probe for label-free selective and sensitive detection of Cu2+ and Fe3+ ions with a limit of detection (LOD) as low as 0.5 nM and 1.0 nM, respectively. The F-g-C3N4 dots were also successfully used to detect Fe3+ and Cu2+ ions in natural water obtained from the Dongpu Lake of Hefei, Anhui province, China.
These findings open up a simple, low-cost route toward green production of F-g-C3N4 dots fluorescent probes for fast, highly selective, and sensitive optical detection of Fe3+ and Cu2+ ions, and show the application potential in drinking water detection.
The related works are published in Nanoscale.
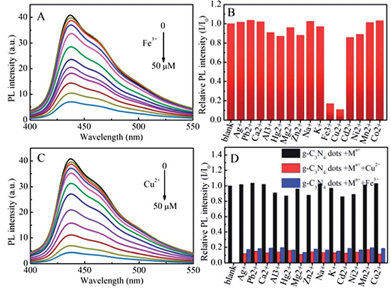
PL intensity changes of F-g-C3N4 dots for different concentrations of Fe3+ (A) and Cu2+ (C). (B) The difference in relative PL intensities of F-g-C3N4 dots between the blank and solutions containing different metal ions. (D) Selective relative PL intensities of F-g-C3N4 dots .
Article links:http://pubs.rsc.org/en/content/articlehtml/2014/nr/c3nr06744k