Recently, new progress in controllable fabrication of nickel nanoparticle chains has been made by the researchers of Fei research group from Institute of Solid State Physics. The related achievement has been published in "J. Mater. Chem. C, 2015, 3, 2072".
It was found that novel collective properties will emerge in the ordered array of nanoparticles (NPs) that is significantly different from the single one. Metallic NP chains as one type of an ordered nanostructure have recently attracted a wide spread attention for their novel properties in optics, electronics, photonic, magnetics and sensing. For example, a noble metallic NP chain was thought to be a promising plasmonic waveguide structure and is expected to be applied in nano-optical waveguide devices. In addition, magnetic NP chains would display significant enhancement of coercivity and ultra-high density data storage ability. It is reported that the performance of the NP chains is strongly related to the particle size, shape and separation. Therefore, controllable fabrication of one dimensional NP chains is very important. To date, various available assembly routes have been used to prepare metal NP chains. Traditional methods containing electron beam lithography and scanning-probe manipulation are suffered from the high cost. The other two usual methods involving self-assembly and Rayleigh instability both have a fatal flaw, that is, the structure parameters of NP chain is hard to control.
In this work, we report a controllable route to fabricate Ni NP chains. Highly uniform Ni NP chain structures consisting of Ni NPs linked with a thin CuO film were prepared by the alternate electrodeposition of Ni/Cu multi-segmented NWs into the pores of an anodic aluminum oxide (AAO) template and subsequently corrosion occurred in the alkali solution. The particle size and separation of the NPs can be adjusted successfully (Figure 1), and the smallest particle size and particle spacing reached to 6 nm and 15 nm, respectively. A series of comparative experiments were carried out to analyze the corrosion mechanism. It is found that the reaction occurred on Ni/Cu Nanowire in NaOH solution is electrochemical corrosion. The electrochemical corrosion in neutral or alkaline solution is an oxygen reduction reaction. A necessary condition for its occurrence is the electrode potential of the metal lower than the potential of the oxygen reduction reaction. For a Ni/Cu NW, Cu segments contact with Ni at both ends, and as a result, there will be a number of contact primary batteries along the axial direction of a Ni/Cu NW. In alkaline solution, the electrode potential of Cu (0.219 v) is lower than the potential of the oxygen reduction reaction (0.41 v), whereas the electrode potential of Ni (0.72 v) is higher than the potential of oxygen reduction reaction; therefore, in each primary battery, Cu as the anode was corroded and Ni as the cathode was undamaged.
This kind of metallic NP chain structure can be applied in the field of sub-wavelength of optical transmission, magnetic memory, drug delivery and so on. The controllable adjusting of the particle size, aspect ratio and particle spacing is important for research of the performance changes under the influence of the geometric parameters.
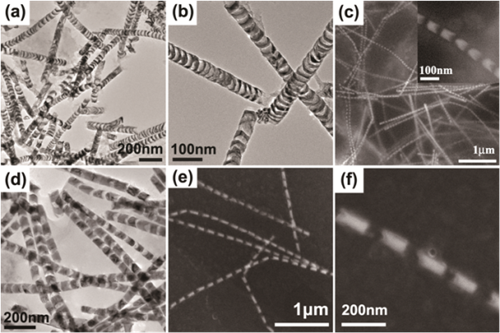
Fig.1 Ni Particle Chain with Different Geometric Parameters
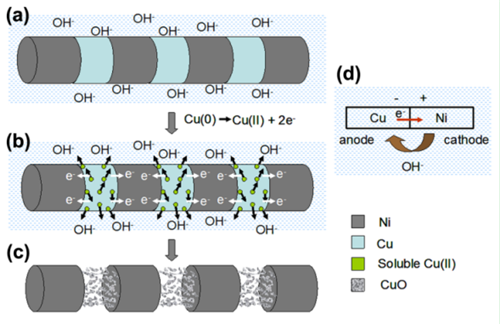
Fig.2 Corrosion Mechanisom of Ni/Cu Nanowire