Nanocatalysts (NCs) have very high catalytic activities due to surface atoms with their unpaired spins. The surface spin arrangements of NCs from disordered to ordered manner under the induction of magnetic fields (MFs) lead directly to the variation of surface energy then influence the catalytic activity of NCs. MFs will be developed as a tool to affect chemical reaction rates if the activity of NCs can be effectively regulated by MFs.
A research group led by Prof. Qianwang Chen in the High Magnetic Field Laboratory, CAS (CHMFL), Hefei National Laboratory for Physical Sciences at Microscale and Department of Materials Science & Engineering of University of Science and Technology of China, found that MFs can increase the rate of 4-nitrophenol (4-NP) catalytic reduction reaction of Pd Nano NCs. The result demonstrated that MFs can reduce the reaction time from 2.6 min to 1.4 min under 0.5 T. Theoretical studies revealed that the magnetic spin configuration (ordered manners) of Pd NCs was in favor of the absorption of 4-NP molecules, and can decrease the system energy.
A chemical reaction catalyzed by certain nanoparticles can be induced or speeded up by altering the strength of an applied MF, which is highly valuable in chemical industry. In addition, it is expected that the increase of MF intensity (> 0.5 T) may lead to more types of nanoparticles to be catalysts and the catalyzed reaction proceeds at a quicker rate.
A schematic diagram of the 4-NP reduction reaction under MFs
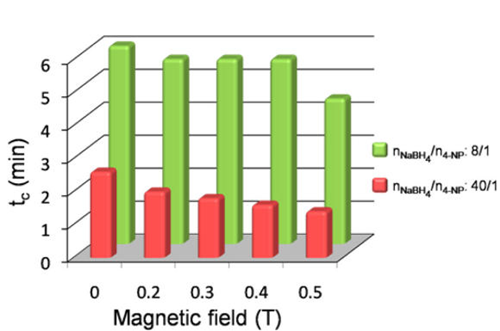
Reaction time (tc) of the 4-NP reduction reaction with the change of the magnetic field strength (T)

Total spin polarized charge density of 4-NP adsorbed on Pd (111) surface without MFs (a) and with MFs (b). The blue region refers to isosurface value of 0.2 e/ Å3.