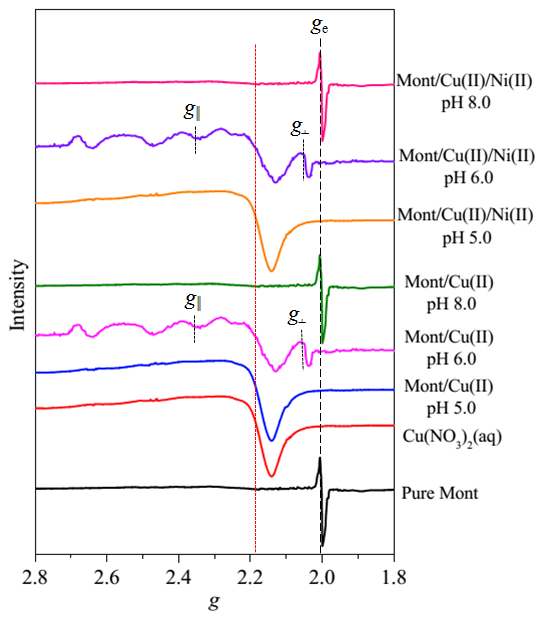
Fig. 1. EPR Spectra of Montmorillonite, Cu(NO3)2(aq) Reference Sample and Cu(II)-Containing Sorption Samples (Imaged by YANG Shitong)
The sorption-desorption, oxidation-redox, complexation- migration, dissolution-precipitation and assimilation-bioaccumulation processes of heavy metal ions in natural environmental system is one of the most basic research content of environmental pollution chemistry. It is crucial to explicitly understand the speciation, migration, transport, bioavailability and ecotoxicity of toxic heavy metal ions in multicomponent environmental systems.
By using the Electron Paramagnetic Resonance (EPR) testing platform in Steady High Magnet Field Facility (SHMFF), research progress in competitive sorption mechanisms of Cu(II) and Ni(II) on natural montmorillonite mineral was achieved by environmental chemistry study team of School for Radiological and Interdisciplinary Sciences, Soochow University.
In view of foregoing environmental background, the study team explored competitive sorption and selectivity sequence of Cu(II) and Ni(II) at montmorillonite/water interface by combining batch experiments, X-ray diffraction (XRD), EPR spectral analysis, surface complexation modeling and X-ray Absorption Spectroscopy (XAS) technique.
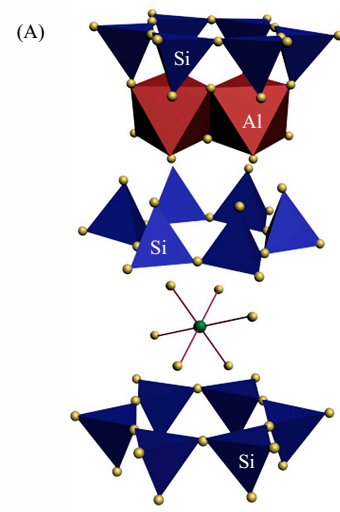 |
|
Fig. 2A. Outer-sphere Complexes
(Imaged by YANG Shitong) |
Findings through EPR analysis indicate that adsorbed Cu(II) species on montmorillonite clearly change in coordination environment with increasing pH values. Specifically, EPR spectrum of montmorillonite/Cu(II) sorption sample prepared at pH 5.0 seems to be similar with Cu(NO3)2(aq) reference sample (Figure 1). This finding suggests that Cu(II) ions are adsorbed by entering montmorillonite interlayer and forming outer-sphere complexes (Figure 2A). Spectrum of sample prepared at pH 6.0 exhibits a distinct anisotropic rigid-limit at g values of ~2.07 and ~2.35 (Figure 1), which provides a strong evidence for occurrence of inner-sphere complexation (Figure 2B) with higher thermodynamic stability than outer-sphere complexes (Figure 2A). For the sorption sample prepared at pH 8.0, complete loss of Cu(II)-induced resonance features in EPR spectrum (Figure 1) points to formation of multinuclear Cu(II) surface complexes (Figure 2C). These EPR findings are well consistent with those deduced through XAS analysis. The derived findings could provide significant information for precise evaluation of fate of the coexisting heavy metal ions in multicomponent environmental systems.
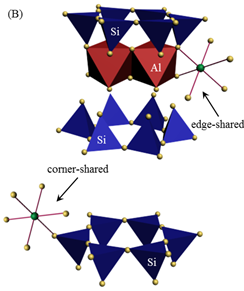 |
|
Fig. 2B. Inner-Sphere Complexes
(Imaged by YANG Shitong) |
Based on fruitful research foundation and superior testing platform, researchers are making unflagging efforts to further explore novel analytical approaches and characterization techniques for clear prediction of speciation and fate of various pollutants in the real aquatic systems. Undoubtedly, continuous studies on these major scientific issues would further promote the fast and steady development of environmental chemistry.
The above findings entitled "Competitive Sorption and Selective Sequence of Cu(II) and Ni(II) on Montmorillonite: Batch, Modeling, EPR and XAS Studies" has been published in the top journal of environmental geochemistry field named Geochimica et Cosmochimica Acta.

Fig. 2C. Multinuclear Surface Complexes (Imaged by YANG Shitong)
Contact:
Prof. YONG Shitong, Ph. D
School for Radiological and Interdisciplinary Sciences (RAD-X), Soochow University
Suzhou, Jiangsu 215123, China
Tel: 0512-65883945
Email:Shitongyang@suda.edu.cn