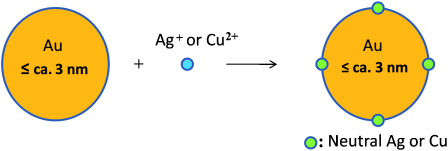
Raise of AGR ( Imaged by LI Manbo)
Galvanic Reduction is a classical reaction named by the Italy scientists Luigi Galvani, which has a history of more than 200 years and has been put into chemistry textbook for middle schools. In 2012, WU Zhikun et al. found that ultra small gold and silver nanoparticles could reduce less noble metal ions, and they suggested term of Anti-Galvanic Reduction (AGR) for the first time (Figure 1).
Recently further research conducted by WU Zhikun’s study team from Institute of Solid State Physics, Chinese Academy of Science has made breakthough on basis of the finding of AGR (Figure 2).
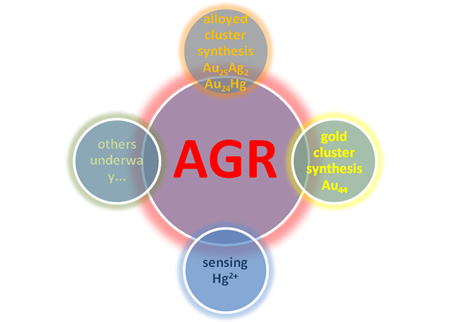
Recent progress of AGR (Imaged by LI Manbo)
About the mechanism, they found that AGR is ion-precursor and ion-dose dependent, which offers novel strategies to tune composition and properties of nanoparticles by varying ion-precursor and ion-dose in AGR reaction (Figure 3A). Considering synthesis application, they prepared two kinds of alloyed nanoclusters Au25Ag2 and Au24Hg with atomical mono-dispersity (Figure 3B and C). Besides, AGR was also employed to synthesize atomically mono-disperse Au44, (Figure 3D). Interestingly, it was found that these nanoclusters synthesized by AGR have unique properties (such as optical, magnetic, catalytic and electrochemical properties, etc). In addition, AGR could be utilized in sensing trace of Hg2+ in environmental samples (including lake water and soil solution), and further more the introduced method exhibit more accuracy than some standard methods (such as ICP-MS) for sensing low-concentrated Hg2+ in lake water (6ppM) (Figure 3E). To be noted, although AGR is opposite to “Galvanic reduction”, it doesn’t violate thermodynamics, and should not be simply attributed to size effect. Further research is underway by WU Zhikun’s study team
Ph. D student YAO Chuanhao, master student TIAN Shubo and assistant professors Man-Bo LI, Nan XIA and Lingwen LIAO all made important contribution to the above study. Some collaborators also provided part of key data and technical support for the study. ESR was conducted in the high Magnetic Field Laboratory of the Chinese Academy of Science (HMFL, CAS) (assisted by Dr. Wei TONG). The studies are financially supported by NSFC (21222301), the ministry of science (2013CB934302), CAS (International innovation team) and Hefei Institute of Physical Science, CAS (2014FXCX002).
The study findings were published in Related results have been published inJ. Am. Chem. Soc. entitledMono-mercury doping of Au25?and the HOMO/LUMO energies evaluation employing differential pulse voltammetry; Nano. Letters entitled Adding Two Active Silver Atoms on Au25 Nanoparticle; Chem. Commun entitled Ion-precursor and ion-dose dependent anti-galvanic reduction and Cu2+ induced formation of Au44(SC2H4Ph)32 and its high catalytic activity for the reduction of 4-nitrophenol at low temperature; Nanoscale entitled Fast, high-yield synthesis of amphiphilic Ag nanoclusters and the sensing of Hg2+ in environmental samples.
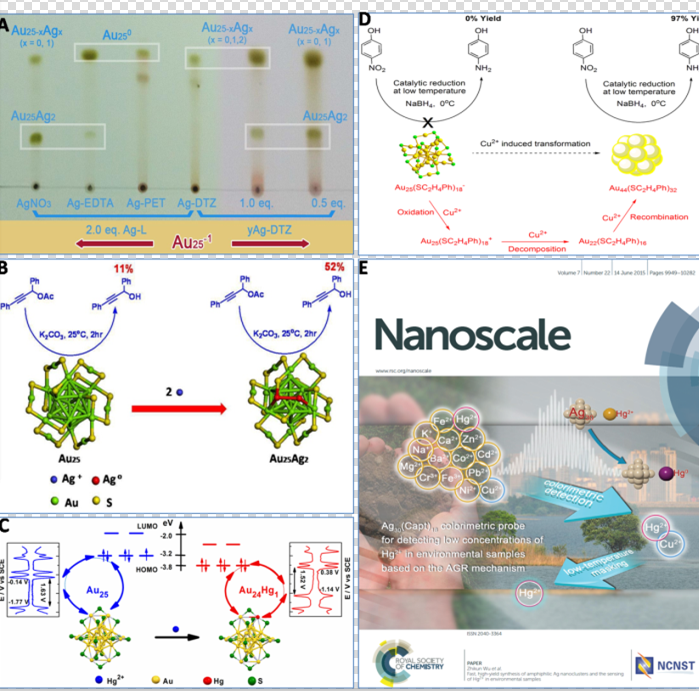
A:Chem. Commun.2015, 51, 11773-11776,B:Nano.Letters.2015,15, 1281-1287,C:J. Am. Chem. Soc.2015, DOI:10.1021/jacs.5b03483,
D:Chem. Commun.2015, 51, 4433-4436,E:Nanoscale 2015, 7, 10013-10020. (Imaged by LI Manbo)
Contact:
Prof. Zhikun WU, Ph.D Principal Investigator
Key Laboratory of Materials Physics, Anhui Key Laboratory of Nanomaterials and Nanostructures
Institute of Solid State Physics, Chinese Academy of Sciences
Hefei, Anhui 230000, China
Tel: 0551-65595395
E-mail: zkwu@issp.ac.cn