With intriguing properties and promising applications compared with mono-metal nanoparticles, bimetal nano-particles remain in frontier for the nanoscience and nanotechnology research. Among various kinds of bimetal nanoparticles, atom-precise and atom-monodisperse ultrasmall nanoparticles (so-called nano-clusters) doped (alloyed) by a second metal which possess insightful structure (composition) property correlation and precise tailoring of properties of bimetal nanoclusters draw extensive attention. However, it remains challenging to control the dopant type, number, and position at atomic level in doped metal nanoclusters via available synthesis methods. Novel methods are in urgent need for precise doping.
In 2012, a study team led by WU Zhikun from Institute of Solid State Physics, Chinese Academy of Science (ISSP, CAS) revealed the unexpected “Anti-galvanic reduction (AGR)”, opposed to the classic “Galvanic reduction (GR)”, which suggests that noble metal (e.g., Au) nanoclusters can reduce some active metal ions (e.g., Ag+) and thus provide a novel method for the precise doping. Then WU Zhikun and his colleagues conducted a series of studies in which inactive metal ions ( locating behind H in the galvanic series ) were generally used. Therefore, several questions naturally arise: Can active metals (e.g., Zn2+, Cd2+) be used in the unique doping method? Can active metals dope nanoclusters? Where would the active metal dopants dopelocated? And what would happen to the property of nanoclusters after doping? Unraveling these secrets are not only interesting and challenging, but also significant in nanocluster and nanomaterials science.
Lately, WU Zhikun joined hands with WENG Honglin from Fudan University and YANG Jinlong from University of Science and Technology of China to conduct a study in which the well defined core-innershell-outershell structured anion Au25(SCH2CH2Ph)18([Au25]- for short) nanocluster and the common divalent Zn2+, Co2+, Ni2+, and Cd2+ were chosen as the matrix (Figure 1) and the active metal ions respectively. The study found that the AGR can occur between [Au25]- and all the investigated cations. Interestingly, Zn2+, Co2+, and Ni2+ can oxidize [Au25]- to neutral or positive species. While Cd2+ can not only oxidize [Au25]- , but also dope to [Au25]-, which was indicated by mass spectrometry and spectrophotometry. Sing crystal X-ray crystallography unravels the structure of the Cd-doped Au25 nanoclusters and revealed that one of theinner-shell gold atoms of Au25 was replaced by a Cd atom.The doping mode is distinctly different from that of mono-mercury doping (J. Am. Chem. Soc.,2015, 137, 9511). Theoretical calculations support the inner-shell cadmium-dopingand indicate that cadmium-doping can greatly tune the electronic structure of Au25, thus result in notable changes of the absorption spectrum of Au24Cd (Figure 2). Additionally, researchers reported that Au24Cd canbe readily transformed into Au24Hg, while the reverse (transformed from Au24Hg to Au24Cd) failed under the investigated conditions (Figure 2). Mechanism of the transformation was primarily explored based on experiment and theoretical calculationsand further investigations are underway.
This study was published in Journal of the American Chemical Society entitled Mono-cadmium vs Mono-mercury Doping of Au25 nanoclusters. This study is sponsored by Natural Science Foundation of China (NSFC) (Nos. 21222301, 21171170, 21401064), the ministry of science (2013CB934302), Chinese Academy of Sciences (International innovation team), Hefei Institute of Physical Science, Chinese Academy of Sciences (2014FXCX002), Hefei Science Center, Chinese Academy of Sciences (user of potential: 2015HSCUP003), etc.

Figure 1. Structure anatomy of Au25 (Image by YAO Chuanhao)
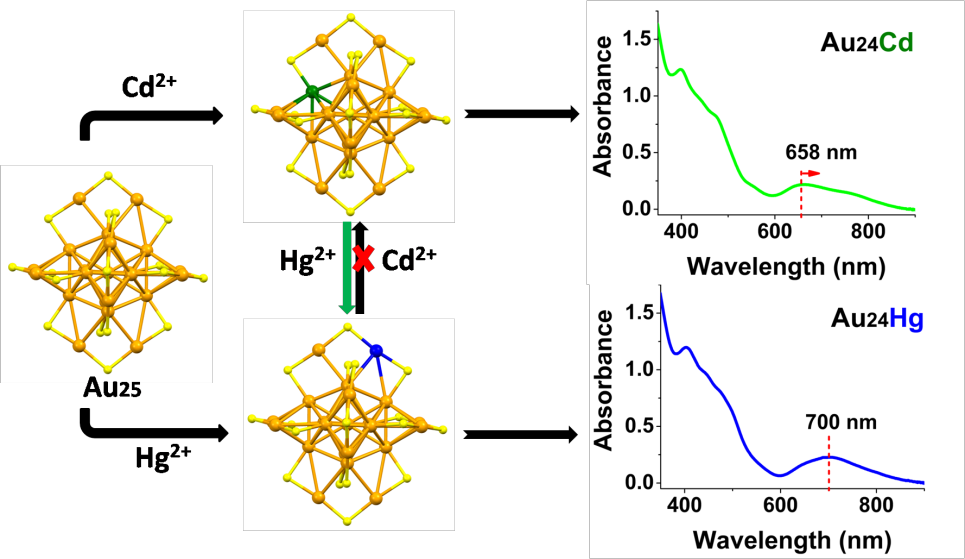
Figure 2. Comparison of mono-cadmium and mono-mercury doping of Au25 (Image by YAO Chuanhao)
Contact:
Prof. Ph.D WU Zhikun, Principal Investigator
Key Laboratory of Materials Physics, Anhui Key Laboratory of Nanomaterials and Nanostructures, Institute of Solid State Physics, Chinese Academy of Sciences
Hefei, Anhui 230000, China
Tel: 0551-65595395
E-mail: zkwu@issp.ac.cn