Due to the excellent properties, such as catalysis, adsorption and magnetism, metal oxide micro/nano structure materials catch attention of researchers and are widely used in electrochemical analysis and determination of heavy metal ions. In fact, electrochemical analysis method study always focuses on realization of high sensitivity and low detection limit. However, the enhancement mechanism of nano-materials remains unclear. In this case, exploring intrinsic impact of different crystal phases in nano-materials through the crystal structure may shed new light on effect of different crystal phases in electroanalysis, which meanwhile benefits developing high efficient sensing interface to determine heavy metal ions.
Given this, Prof. LIU Jinhuai, 973 chief scientist, and Prof. HUANG Xingjiu from Institutes of Intelligent Machines, Chinese Academy of Sciences (CAS) joined force with Prof. HUANG Yuying and Associate Prof. LI Lina from Shanghai Synchrotron Radiation Facility, Shanghai Institute of Applied Physics, CAS to conducted a study in which they revealed the electrochemical analysis mechanism on different crystal phase of nano-Fe2O3 by advancing approach extended X-ray absorption fine structure (EXAFS). The study results in a paper which was published in journal of Analytical Chemistry with the title of Iron Oxide with Different Crystal Phases (α- and γ-Fe2O3) in Electroanalysis and Ultra-Sensitive and Selective Detection of Lead(II): An Advancing Approach Using XPS and EXAFS.
In the study, researchers explored the influence of different crystalline phases Fe2O3 (α- and γ-Fe2O3) nano-flower on the electrochemical detection of Pb(II). Considering the crystal structure with adsorption capacity and adsorption methods, they unveiled the relationship between electrochemical phenomenon and crystal structure. The findings show that as for Fe2O3 nanoflowers electrochemical stripping behaviors toward Pb(II) vary with crystal phases. The sensitivity of γ-Fe2O3 modified electrode is about three orders of magnitude higher than that of α-Fe2O3 modified electrode (Figure a).
In addition, researchers proposed evidence of X-ray photoelectron spectroscopy (XPS) and EXAFS to verify the difference in local structural environment of the adsorbed Pb(II) on the surface of α- and γ-Fe2O3. XPS results suggest that surface of γ-Fe2O3 is covered with more hydroxyl groups due to the existence of vacant cation sites (Fig. a) which usually occurs in octahedral positions and is recognized as one of the crystal defect. These surface hydroxyl groups on the iron oxides can exchange with Pb(II) to form surface complexes resulting in the higher adsorption capacity of Pb(II) than that of α-Fe2O3.
Furthermore, EXAFS indicates that α- and γ-Fe2O3 can diversely interact with Pb(II) and iron (hydr)oxides respectively. While bonded only as Pb-OH on surface of γ-Fe2O3, Pb(II) ions adsorbed on the surface of α-Fe2O3 are bonded both as Pb-OH and Pb-H2O.(Figure c and d) This study not only sheds new light on sensitive electro-analysis using micro-materials modified electrode, compared to the state-of-the-art electro-chemical methods for detection of heavy metal ions, but lays a foundation for a high efficient sensing interface to determine other heavy metal ions (not only Pb(II)) by choosing different crystal structures.
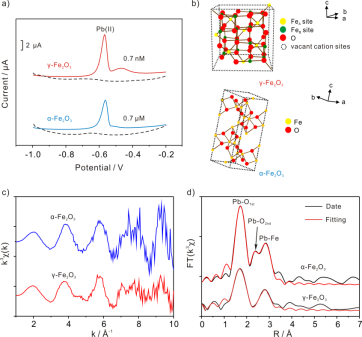
a, Typical SWASV responses of Pb(II) on α-Fe2O3 and γ-Fe2O3modified GCE (glassy carbon electrode) at different concentrations. b, Graphical representations of the fundamental crystal of α-Fe2O3 and γ-Fe2O3. c, Normalized, k3-weighted Pb LIII-XAFS spectra of the Pb(II)/α-Fe2O3 and γ-Fe2O3. d) Fourier transforms (FTs) of EXAFS data with fits to spectra uncorrected for phase shift. (Image by LI Shanshan)
Contact:
Prof. HUANG XingJiu, Ph.D Principal Investigator
Nanomaterials and Environmental Detection Laboratory, Institutes of Intelligent Machines, Chinese Academy of Sciences
Hefei 230031, Anhui, China
E-mail: xingjiuhuang@iim.ac.cn (X.J.H)
Tel.: +86-551-65591142; fax: +86-551-65592420