Electrochemical techniques are extensively used as a rapid, cheap, and portable method in arsenic determination. According to previous literature, various modified electrodes apply to analysis of arsenic (As(III)-specific ligands, enzymes, carbon nanotubes (CNTs), graphene) and noble metals (gold silver and platinum). And Au is considered a priority as the working electrode of the solid metal substrates. In fact, it is not easy to obtain and repeat construction of gold nanostructures with well controlled morphology on electrodes, which is due to both the use of surfactants during the preparation process and the surface tension of the bare electrode during modification. And there is no doubt that at present it is not very difficult to prepare the materials with regular morphology. However, a uniform and controllable distribution of prepared nanomaterials on the surface of electrodes are complex. Moreover, the surfactant of nanoparticles would directly affect electrochemical performance. In this case, a simple-fabricated electrode which possesses outstanding arsenic detection performance but needs no extensive morphology control remains in urgent need.
Targeting this problem, Prof. LIU Jinhuai, 973 chief scientist, and Prof. HUANG Xingjiu from Institute of Intelligent Machines (IIM), Chinese Academy of Sciences (CAS) manage to design and fabricate a sensing interface for ultra-sensitive detection of As (III) by abundantly dispersing Au nanoparticles on the surface of Fe3O4 nanosphere. The progress was published in journal of Analytical Chemistry with the title of Adsorbent Assisted In Situ Electrocatalysis: An Ultra-Sensitive Detection of As(III) in Water at Fe3O4Nanosphere Densely Decorated with Au Nanoparticles.
Inspired by the excellent catalytic properties of micro-sized Au nanoparticles and the good adsorption ability of the Fe3O4 nanosphere toward arsenic, researchers report the concept of adsorbent-assisted in situ electrocatalysis for ultra-sensitive detection of As(III) on the Fe3O4 nanosphere which is densely decorated with Au nanoparticles. Au NPs and Fe3O4 jointly enhance the sensitivity of arsenic detection without morphology control. The redox of As(III) occurs with direct catalysis on the Au NPs surface in situ without diffusing to SPCE after being adsorbed by Fe3O4 nanosphere (Figure).
In addition, according to the theory of diffusion, the inequality d > 2{2D(ΔE/v)}1/2 avoids diffusion zone overlap (known as shielding effects). The inequality I2/2I1=95% with d/r>20 is employed, where I2 is the steady-state diffusion-limited current at two interfering coplanar microspheres, and I1 is diffusion-limited current at two independent coplanar microspheres. Keeping d > 20r is necessary for individual independent diffusion. If not, the adjacent diffusion layers overlap and seriously decrease values of d. When d is far below 20r, there is complete overlap and a linear concentration profile like the diffusion of a macrodisc. In the system, the mean distance is 2.6nm versus the mean diameter of 5.4nm Au particle. It is clear that the separation distance between adjacent Au nanoparticles will cause heavily overlapping diffusion layers and subsequently generate a voltammetric response equivalent to a gold macrodisc electrode as what was found in gold nanoparticle-modified glassy carbon microspheres.
Through this new design, good morphology control can be ignored, which is a sharp contrast to the previously developed pure gold nanoparticle-modified electrodes. It is expected to design a highly efficient sensing interface for heavy metal ions determination.
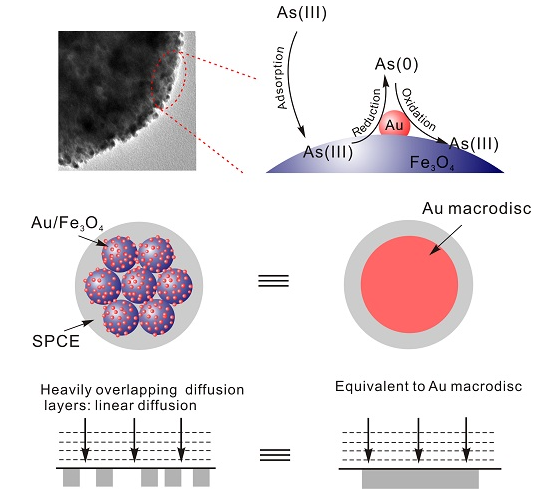
Figure. Schematic of adsorbent assisted In situ electrocatalysis for As(III) detection based on chemical architectural design taking advantage of the synergy between electrocatalysis of Au NPs and adsorption of Fe3O4 nanosphere at Au@Fe3O4-RTIL SPCE. (Image by LI Shanshan)
Contact:
Prof. HUANG XingJiu, Ph.D Principal Investigator
Nanomaterials and Environmental Detection Laboratory, Institutes of Intelligent Machines, Chinese Academy of Sciences
Hefei, Anhui 230031, China
Tel.: +86-551-65591142
fax: +86-551-65592420
E-mail: xingjiuhuang@iim.ac.cn