
In a recent study published in Nature Chemistry, scientists reported the synthesis of a novel nitride with metallic luster and hexazine rings—the result of a six-year effort in high-pressure science.
This is the first time that a planar N62- , a dianionic hexazine nitride, has been obtained in a laboratory experiment. Furthermore, the structure remained relatively stable at pressures down to 20 GPa.
Nitrogen-rich compounds have attracted wide attention because of their great potential as high-energy density materials (HEDMs) that can store and release huge amounts of energy. However, very few nitrogen compounds have been synthesized so far in comparison with the number predicted theoretically through calculation and modelling.
"Low-order N-N bonds are hard to keep stable at low pressure," said Dr. WANG Yu, lead author of the study and a researcher at the Hefei Institutes of Physical Science (HFIPS) of the Chinese Academy of Sciences. According to WANG, that is why synthesizing nitrogen compounds in the laboratory is so difficult.
In previous studies, WANG and her colleagues learned that molecular diatomic nitrogen can be converted into an atomic solid with a single-bond crystalline cubic gauche (cg-N) structure in a diamond anvil at extreme pressures up to 110 GPa and 2,500 K. The result inspired amount of synthesis of polynitride materials at high pressure and high temperature, including the results in their experiments.
However, how to maintain compound stability and restore species under practical conditions still remains challenging.
In the current study, the researchers decided to use alkali metals that have been shown to reduce the pressure required for synthesis, thus improving the stability and energy density of potential HEDM compounds.
Scientists had also learned that using linear N3- groups as precursors not only significantly reduces the activation barrier, but also provides a more uniform reaction environment.
The researchers thus began their synthesis using alkali metals and linear N3- azides. They began with investigation of potassium azide (KN3), which they placed in a diamond anvil chamber used for X-ray diffraction and Raman experiments.
Through trial and error, the scientists synthesized the N62- plane in the laboratory and stabilized the newly synthesized structure in K2N6. The successful synthesis was based on KN3 heated by a laser while in a diamond anvil cell at pressures above 45 GPa.
Using X-ray diffraction and Raman detection results, the researchers, in collaboration with a team led by Artem Oganov at Skolkovo Institute of Science and Technology, found that all their results were consistent with theoretical predictions through first-principles calculations.
WANG said the researchers were "are all very excited" to achieve a nitride with N62 hexazine rings for the first time in a lab experiment.
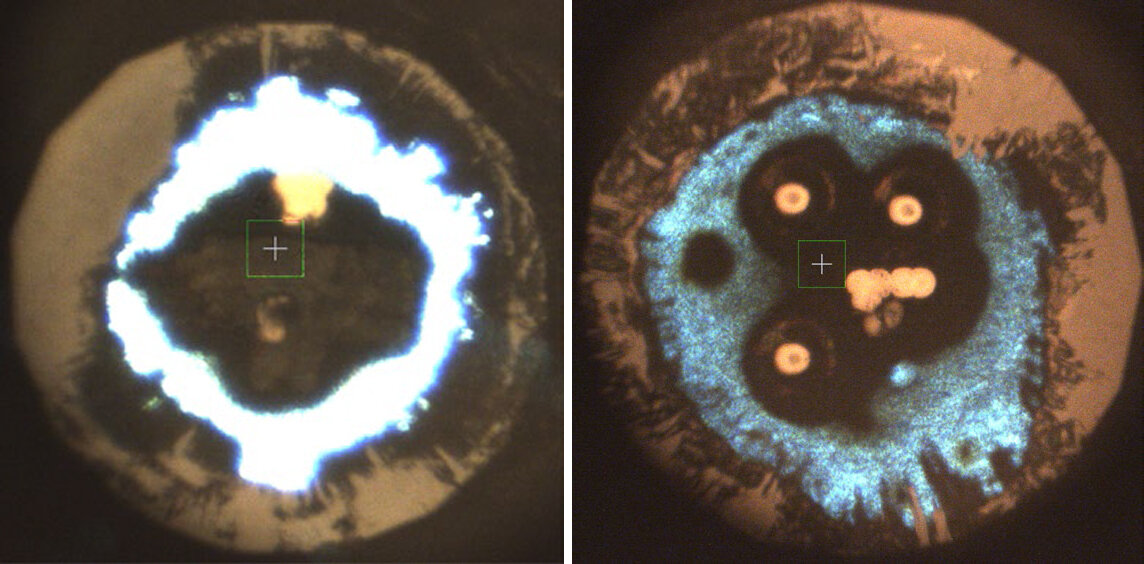
Microphotographs of laser-heated potassium azide samples at pressures of 500,000 atmospheres (left) and 300,000 atmospheres (right). The white to light-blue areas on the outside are K1N3. Toward the center, the material has transformed into K2N6 in the left photo and a mysterious and poorly understood compound with the formula K3(N2)4 on the right. (Image by WANG Yu)