
Recently, a collaborative research team led by Professor HUANG Xingjiu from the Hefei Institutes of Physical Science of the Chinese Academy of Sciences and Professor LI lina from Shanghai Institute of Applied Physics of the Chinese Academy of Sciences, successfully reveals the selective catalytic double metal single atom of heavy metal redox reaction via combined high-throughput calculation and in situ characterization technology.
The results have been published in Nano Letters.
"This new method allows us to observe and understand how the bimetallic single-atom catalyst changes while it's working," explained Dr. SONG Zongyin, a member of the team, "The catalyst can be used to detect harmful heavy metals in the environment, like copper and arsenic."
Current limitations in spatial and temporal resolution restrict our understanding of atomic-level microscopic dynamics, which hinders the development of catalyst regulation technology and its broader application. To address this, the research group focuses on designing high-performance, sensitive materials for the precise detection of environmental pollutants and body fluid electrolyte ions.
In this study, the researchers combined the advanced in situ synchrotron radiation spectroscopy technology and the high-precision theoretical calculation to realize the real-time capture and analysis of the transient structure of the bimetal single-atom catalyst in the catalytic reaction process. Through high-throughput screening, they identified an efficient duplex metal atomic electrode interface that enables the parallel electrochemical reduction of Cu(II) and As(III).
Moreover, in situ X-ray absorption fine structure (XAFS) spectroscopy, along with coordination field theory, verify the NiCu specific level matching promoted by the permissive dd transition in bimetallic single-atom systems. This approach enabled the reconstruction of the electrochemical dynamic reduction process at the atomic level.
Density functional theory (DFT) calculations revealed that the Fe-As specific bonding and minimum potential energy determination step is the linear shift of the main s and p peaks to the high-energy orbit derived from the key intermediates. The dynamic evolution of adaptive matching was reproduced from the perspective of the dynamics, the convergence trend of the thermodynamic model, and the annealing simulations, respectively.
The study of reaction mechanisms helped optimize catalyst performance, while experimental verification confirmed predictions and revealed the catalyst's structure-performance relationship.
The combined approach of in situ characterization and theoretical simulation provides unique insights for the investigation of transient reaction dynamic mechanisms and future selective design/screening of nextgeneration catalysts, according to the team.
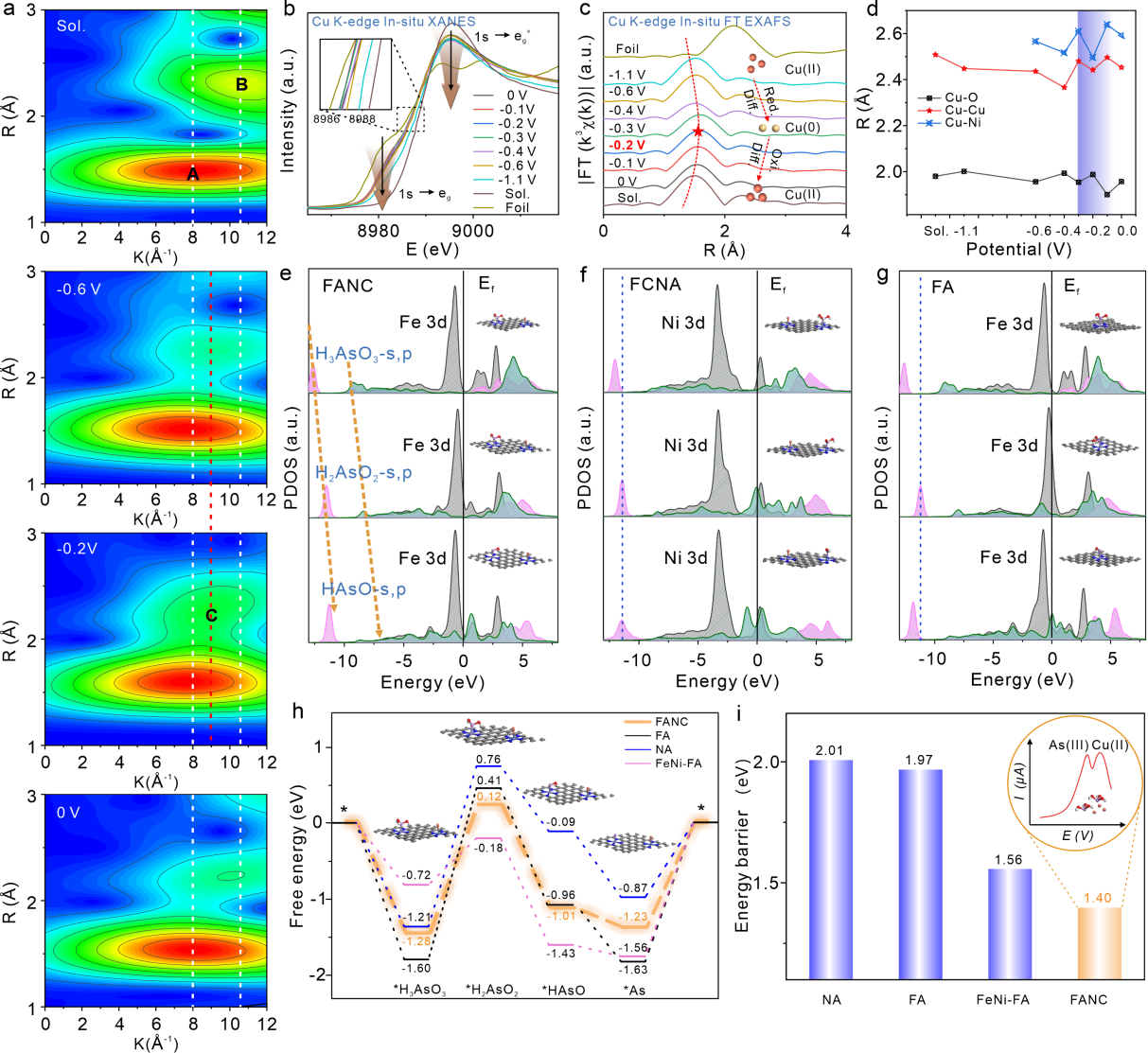
In-situ XAFS technology combined with DFT computational simulation to explore the microscopic dynamic evolution of electrochemical reactions. (Image by SONG Zongyin)