
In a recent study published in Science China Life Sciences, a research team led by WANG Junfeng at the Hefei Institutes of Physical Science of the Chinese Academy of Sciences introduced an innovative approach to developing high-affinity receptor-containing antibodies in vitro, offering a promising strategy to combat malaria.
Malaria is a serious infectious disease caused by Plasmodium parasites and spread by mosquitoes. To evade the host immune system, the parasite expresses variant surface antigens like RIFINs, which bind to immune-inhibitory receptors such as LILRB1 and LAIR1. Recently, scientists have discovered a rare class of “receptor-containing antibodies” in malaria patients. These antibodies incorporate domains from inhibitory receptors, allowing them to block the interaction between RIFINs and host receptors, disrupting immune evasion. However, they are rare, hard to isolate, and have low binding affinity, limiting their clinical potential.
In this study, the team employed a structure-guided affinity maturation strategy. They first engineered a high-affinity receptor fragment variant and then grafted it into the framework of MDB1, a known human anti-malaria antibody. This process yielded a new antibody, D1D2.v-IgG, which exhibited a 30-fold higher binding affinity to the malaria antigen RIFIN#1 (PF3D7_1254800) compared to native LILRB1.
Functional assays confirmed that D1D2.v-IgG could specifically recognize RIFIN#1 on both K562 cells and Plasmodium-infected red blood cells, effectively neutralizing the parasite’s immune evasion.
Building on this success, the researchers designed a bispecific antibody called NK-biAb by fusing a single-chain antibody (scFv) with NKG2D, a receptor found on natural killer (NK) cells. This engineered antibody not only blocked RIFIN#1-mediated immune evasion but also actively recruited NK cells to attack the infected cells, significantly enhancing immune cell-mediated cytotoxicity.
This study provides a novel strategy for developing antibodies targeting Plasmodium falciparum immune evasion mechanisms and opens new avenues for innovative antimalarial drug discovery.
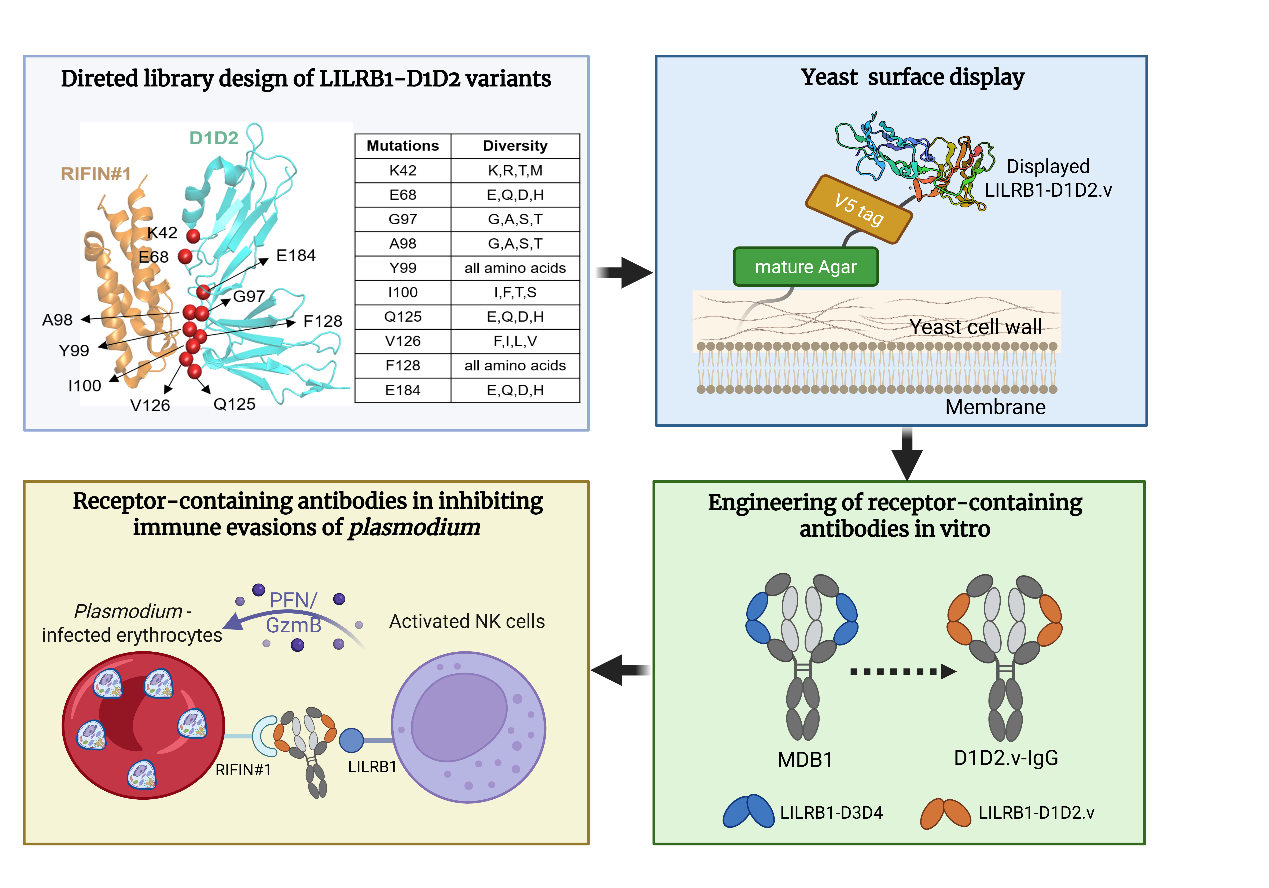
Structure-based engineering and functional characterization of D1D2.v-IgG for inhibiting RIFIN#1-mediated immune evasions (Image by WANG Junfeng)