
Recently, a research team from the Hefei Institutes of Physical Science of the Chinese Academy of Sciences, have constructed a copper single atom catalyst (Cu-N3 SAs) with an N coordination structure using two-dimensional g-C3N4 derived from melamine pyrolysis as a carrier, achieving efficient electrocatalytic urea synthesis under mild conditions.
The research results were published in Angewandte Chemie International Edition.
Urea is mainly synthesized via the energy-intensive and highly polluting Bosch-Meiser process. Therefore, it is crucial to develop sustainable urea synthesis methods driven by clean energy. However, the synthesis of urea via the electrocatalytic co-reduction of CO2 and NO3– still faces many challenges: multi-electron reaction processes, complex C-N coupling reaction mechanisms, and competitive side reactions, all of which greatly reduce the performance of urea synthesis.
In this work, the researchers employed two-dimensional g-C₃N₄, derived from melamine pyrolysis, as a carrier to stabilize copper atoms in a Cu–N₃ coordination structure. Using a tandem impregnation–pyrolysis method, they constructed copper single-atom electrocatalysts (Cu–N₃ SAs). Advanced characterization techniques, including X-ray absorption fine structure (XAFS) and X-ray photoelectron spectroscopy (XPS), confirmed the precise atomic structure and electronic state of the catalysts.
The Cu–N₃ SAs demonstrated remarkable activity, achieving a urea yield of 19,598 ± 1,821 mg h⁻¹ mgCu⁻¹ and a Faradaic efficiency of 55.4% at –0.9 V (vs. RHE). Further insights from in-situ infrared spectroscopy, mass spectrometry, and X-ray absorption spectroscopy revealed that under reaction conditions, the Cu–N₃ sites dynamically reconstruct into an N₂–Cu–Cu–N₂ configuration, which significantly boosts urea synthesis performance.
Complementary density functional theory (DFT) calculations showed that this reconstruction occurs within the ring structure of single-layer g-C₃N₄. The resulting copper bisite structure enhances CO adsorption, accelerates multi-electron transfer, and lowers the energy barrier for the crucial *CONH intermediate formation—the first C–N coupling step in urea production.
According to the team, this study provides important theoretical guidance for understanding the dynamic evolution of actual catalytic active sites in efficient urea electrolysis.
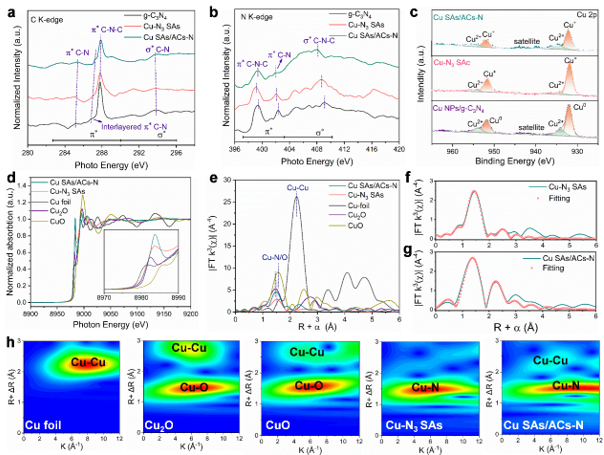
Structural characterizations of series catalysts. (Image by ZHANG Shengbo)
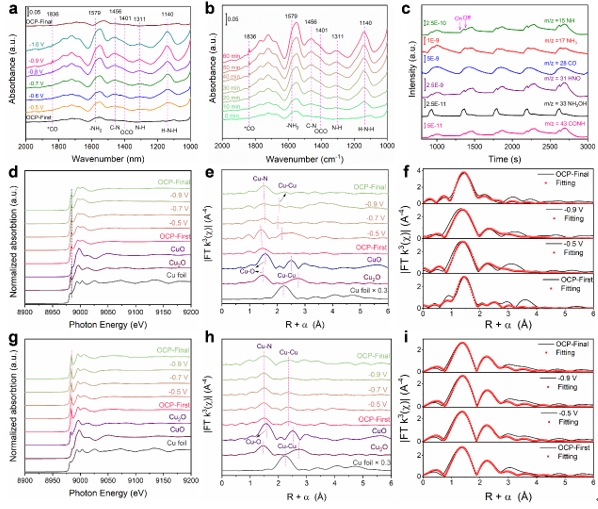
Openrado electrochemical spectroscopy and differential mass spectrometry measurements. (Image by ZHANG Shengbo)