
Novel ways were found to enhance the stability of diamane synthesized by high-pressure method, according to a research team led by Prof. WANG Xianlong from Solid State Physics, Hefei Institutes of Physical Science (HFIPS) of Chinese Academy of Sciences (CAS).
The related results were published in Physical Review B.
"Though introducing B and N dopant into diamane, we found that the structural and electronic properties of diamane could be regulated," said NIU Caoping, first author of the paper.
Diamane is a two-dimensional single-layer diamond obtained by compressing bi-layer graphene to form interlayer sp3 bonds. With the characteristics of both graphene and diamond, it is expected to be a new two-dimensional carbon material alongside graphene. However, the diamane synthesized by high-pressure method is converted back to graphene after releasing pressure, and it cannot be maintained under ambient conditions.
In this research, researchers investigated the structural and electronic properties of different doped forms of B and N atoms in cubic and hexagonal diamane based on the first-principles methods.
They found that the doping configuration reduced the formation energy of diamane and promoted the synthesis of diamane, which enhanced the stability of diamane at ambient condition.
Researchers proposed an easiest mechanism to synthesize diamane experimentally by compressing bi-layer graphene with one layer doped with B atom and the other doped with N atom.
Diamane doped with N atoms was easy to achieve experimentally because the formation energy of diamane was not sensitive to the distribution of N dopant. In addition, depending on the concentrations and distributions of B and N atoms, doped diamane had variable electronic structures (semiconductor, metal, superconductivity ~ 4 K), which could be applied to the field of two-dimensional electronic devices.
This work proposed a new scheme to synthesize more stable and functional diamane.
This work was supported by the National Natural Science Foundation of China, and the calculations were performed in the Center for Computational Science of the Hefei Institutes of Physical Science of the Chinese Academy of Sciences.
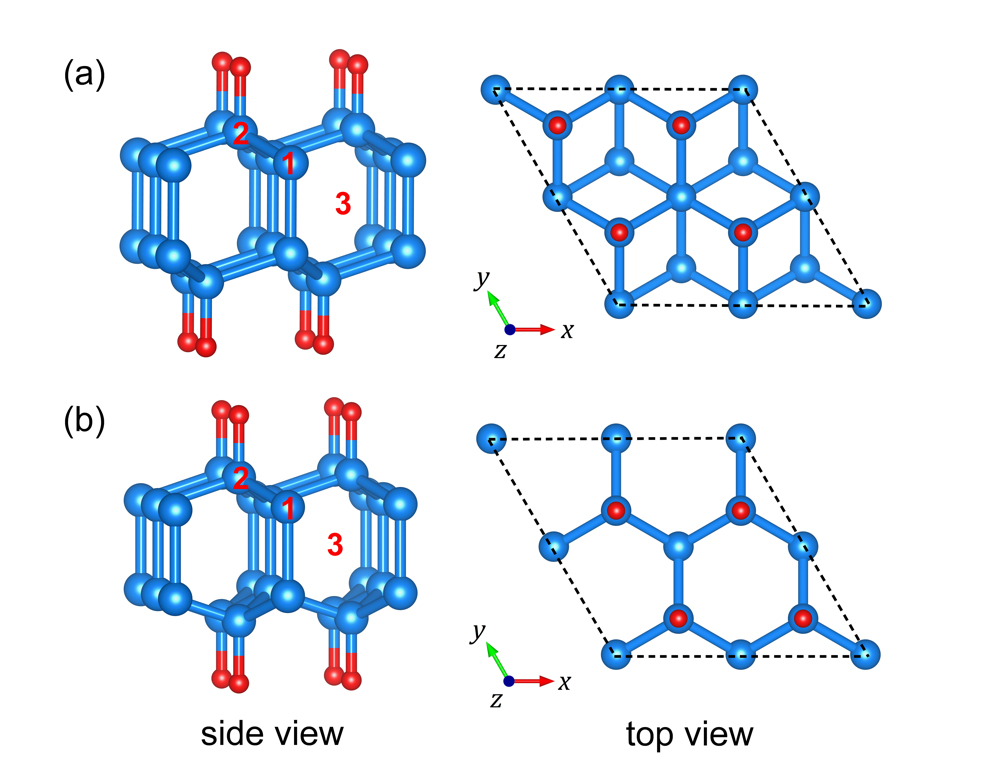
Fig. 1 Configurations of cubic diamane (a) and hexagonal diamane (b). (Image by NIU Caoping)
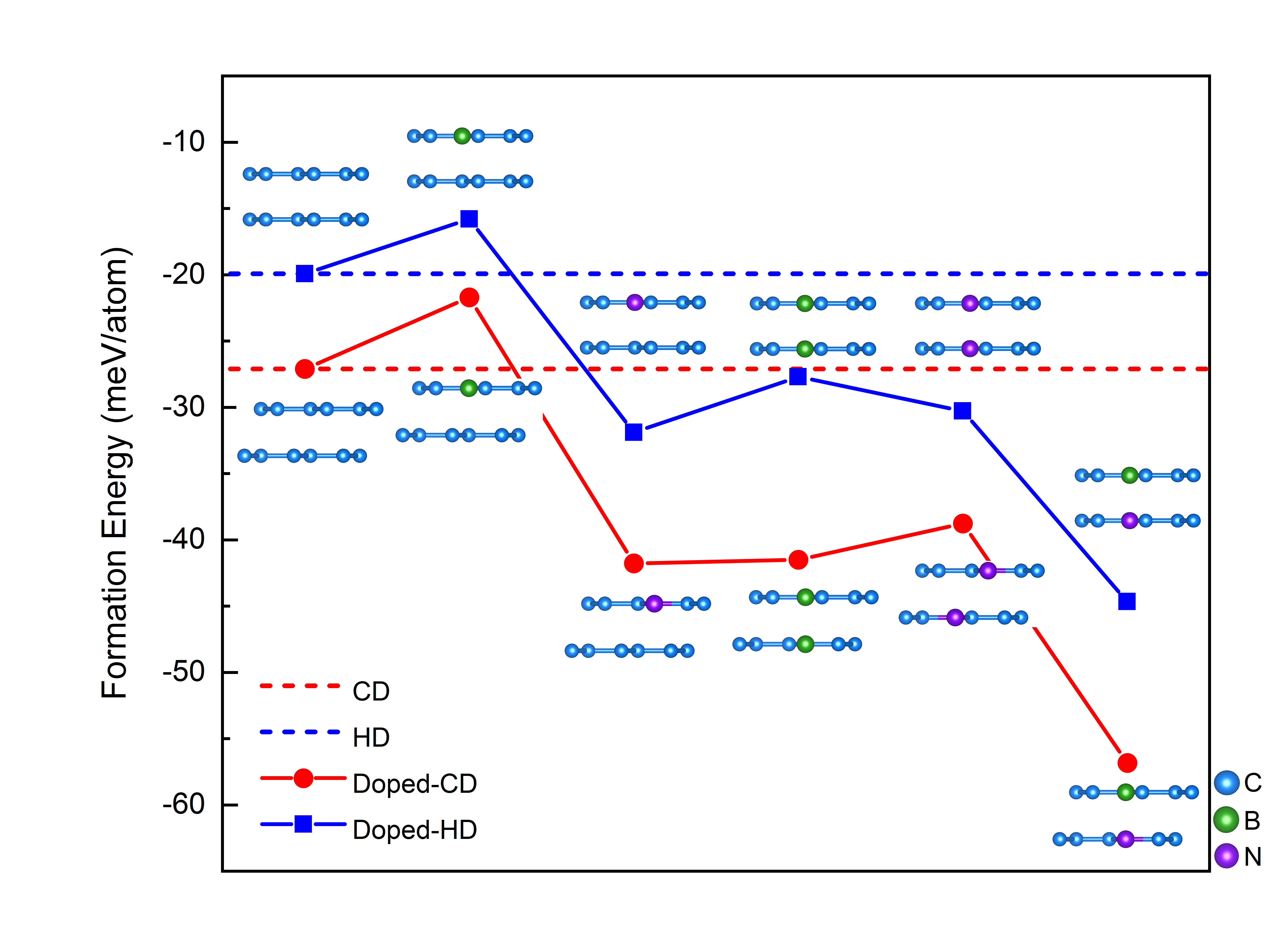
Fig. 2 Formation energies of diamane with different doping methods at the concentration of 6.25 mol%. (Image by NIU Caoping)

Fig. 3 The bandgaps of different doping configurations. Bars with simple color represent the direct bandgap, and bars with slanted lines on the surface represent the indirect bandgap. The dashed red and blue lines show the bandgap of pristine cubic and hexagonal diamane, respectively. (Image by NIU Caoping)