
Recently, a research team from the Institute of Plasma Physics, the Hefei Institutes of Physical Science of the Chinese Academy of Sciences, successfully developed an engineered Escherichia coli strain capable of efficiently producing 3-FL, achieving a substantial increase in yield.
The research findings have been published in the Journal of Agricultural and Food Chemistry.
3-Fucosyllactose is an essential component of human milk oligosaccharides and plays an irreplaceable role in infant health. However, due to the immense difficulty of extracting 3-FL directly from breast milk, its biosynthesis has long been a hotspot and challenge in scientific research.
In this study, the research team used E. coli BL21 Star as the host. The research team employed multiple optimization strategies to construct a strain with high 3-FL productivity.
They first integrated a 3-FL production module into E. coli and screened for an efficient α-1,3-fucosyltransferase. Further improvements were achieved by optimizing the genomic integration sites of key genes and enhancing precursor pathways and transport systems.
Through a series of genetic engineering modifications and fermentation condition optimizations, the high-yielding strain 3FL05-1 was obtained. In shake-flask fermentation, the strain produced 11.26 g/L of 3-FL. Upon scale-up in a 5-liter bioreactor, the yield increased significantly to 60.24 g/L, with a lactose conversion rate of 68% and productivity of 0.19 g/L/h—representing a new record in reported microbial 3-FL production.
This achievement not only provides a new technological way for the industrial production of 3-fucosyllactose but also offers valuable experience and reference for the biosynthesis of other human milk oligosaccharides.
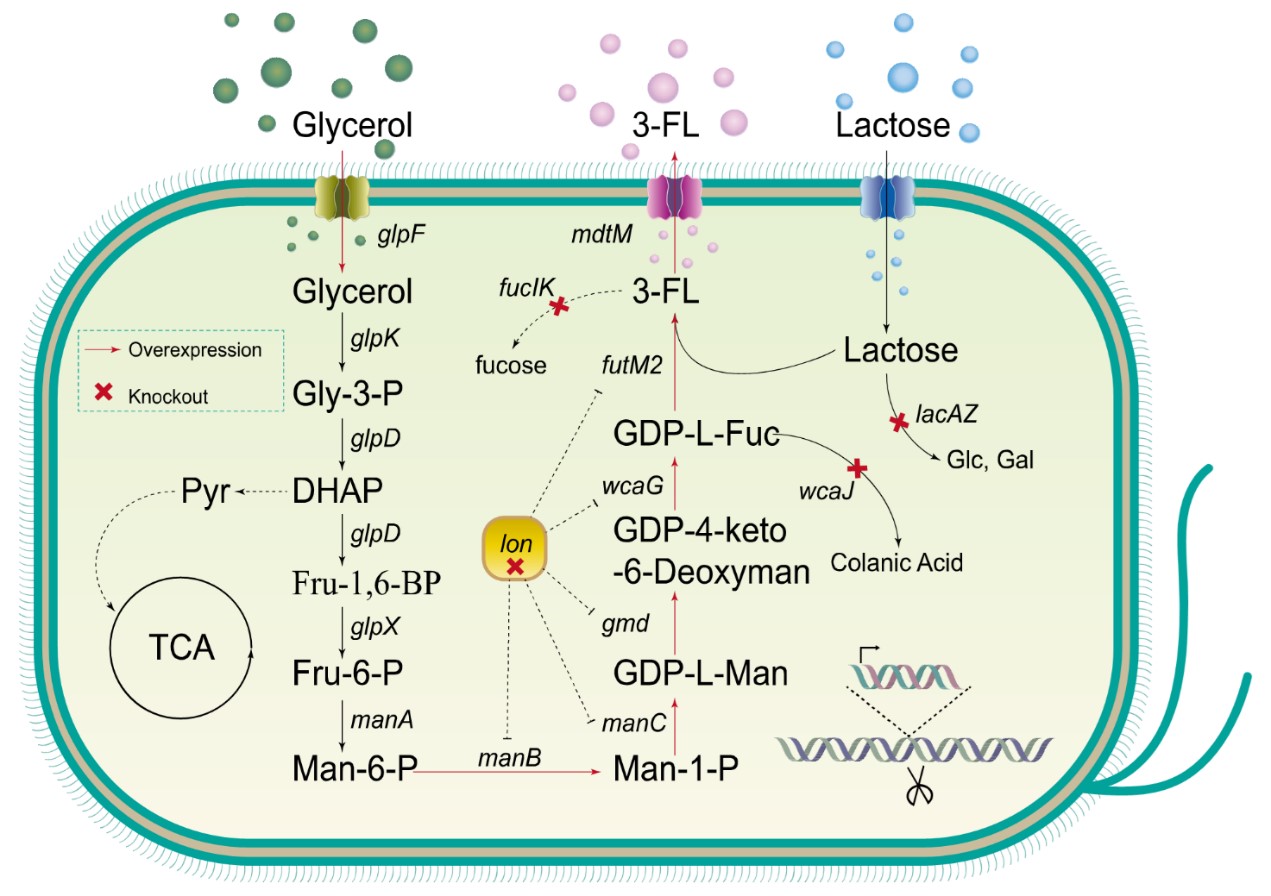
De Novo Biosynthetic Pathway of 3-Fucosyllactose in Escherichia coli. (Image by LU Shujie)
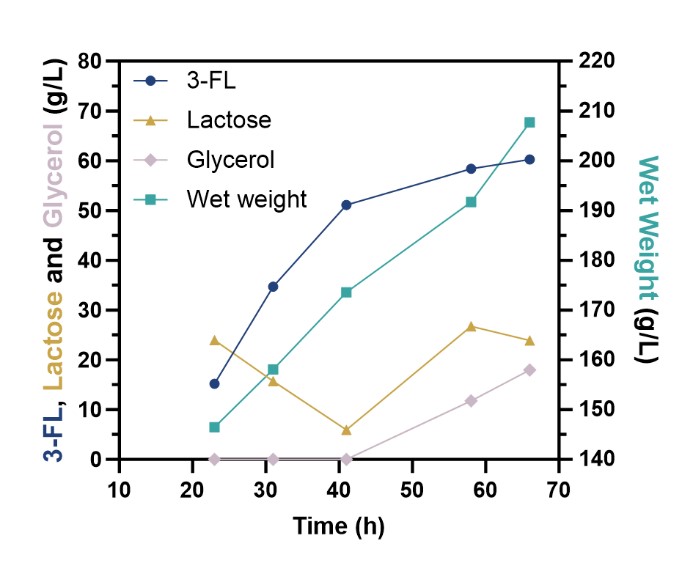
Fermentation of 3-Fucosyllactose in a 5 L Bioreactor (Image by LU Shujie)