
Recently, the research team led by Prof. LIANG Changhao from the Institute of Solid State Physics, the Hefei Institutes of Physical Science of the Chinese Academy of Sciences, has demonstrated a method for the synthesis of carbon nanotubes (CNTs)-supported intermetallic RuM (M = Cu, Rh, and Pd) alloys dominated by sub-5 nm nanoparticles (NPs).
The related research results were published in Advanced Science.
Sub-5 nm Ru-based alloys have emerged as excellent electrochemical catalysts for water splitting. However, their synthesis is limited by thermodynamic immiscibility, which poses a significant challenge to the creation of a widely applicable preparation method.
In this study, the team employed nanosecond laser ultrafast confined alloying (LUCA) to overcome the immiscible-to-miscible transition limit in the synthesis of CNTs supported sub-5 nm bimetallic RuM (M =Cu,Rh, and Pd) alloy NPs.
The alloying of non-noble metal Cu with varying atomic ratios of RuCu alloys is appealing owing to the low price of Cu and cost-effective synthesis for large-scale practical applications.
The team's research demonstrated that the Ru95Cu5/CNTs catalysts displayed excellent electrocatalytic alkaline hydrogen evolution reaction (HER) activity with an overpotential of 17 mV and Tafel slope of 28.4 mV dec−1 at 10 mA cm−2.
Furthermore, the catalyst showed high robustness over long-term 5000 cyclic voltammetry cycles. The performance is much better than LUCA-synthesized CNTs-supported Ru86Rh14,Ru89Pd11, Ru, and Cu NPs catalysts, as well as commercial benchmark 20% Pt/C and other mainstream Ru-based catalysts including wet chemistry-synthesized RuRh particles (overpotential of 25 mV, Tafel slope of 47.5 mVdec−1) and RuCu/CNTs (overpotential of 39 mV) synthesized using the flash Joule heating method.
This research highlights the great potential of LUCA for screening new classes of HER catalysts.
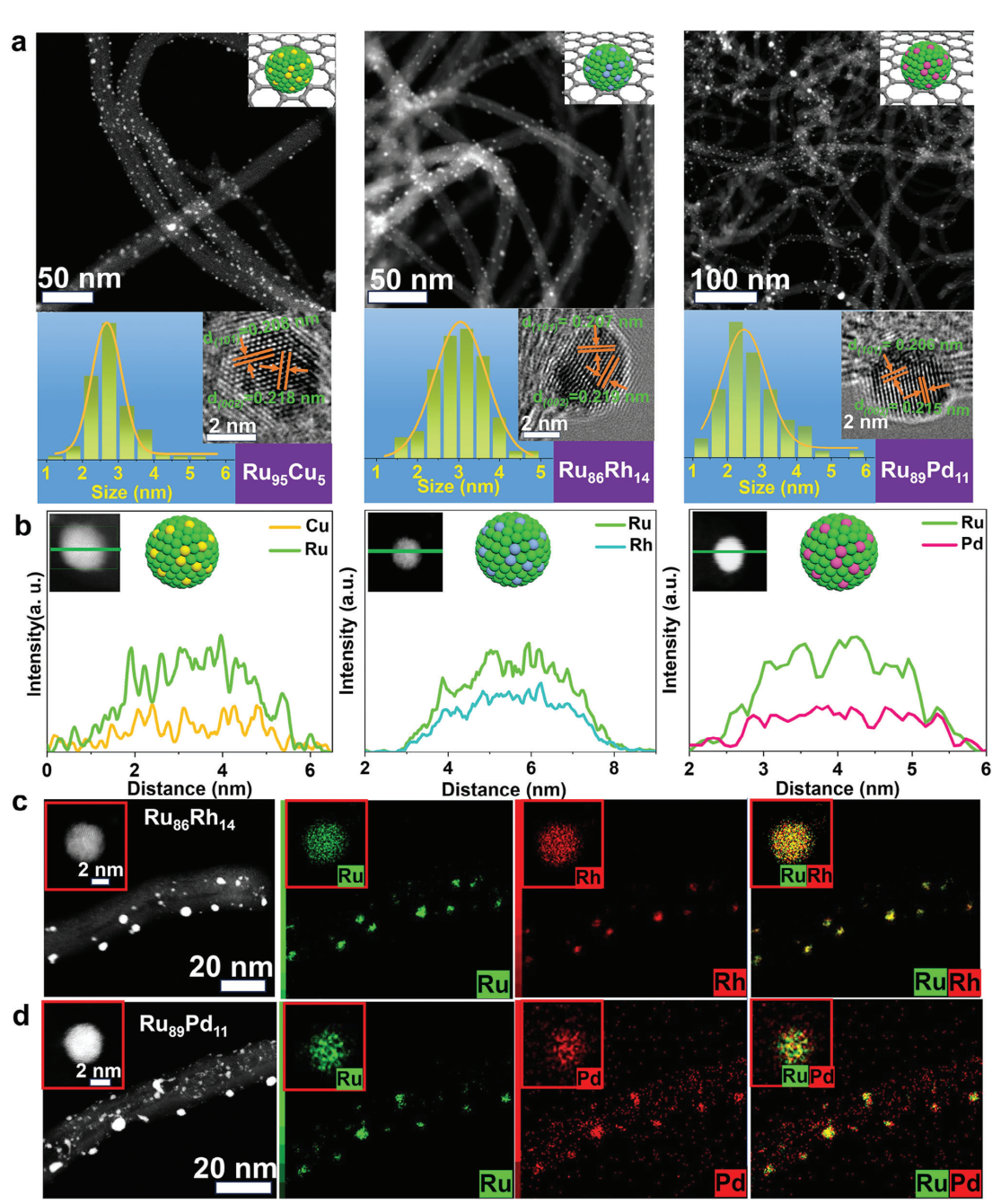
RuM/CNTs (M = Cu, Rh, and Pd) composites size/composition (Image by YE Yixing)