
A research team led by ZHANG Fan at the Hefei Institutes of Physical Science of the Chinese Academy of Sciences, working with Professor HUANG Zhiwei' s group at Harbin Institute of Technology, has mapped a core immune network that drives the progression of hepatitis B.
Their study, recently published in Science Bulletin, reveals how distinct immune cell interactions shape disease stages and suggests new targets for therapy.
HBV remains a global health challenge, particularly in Asia and Africa. While adults typically clear the virus, early-life infections often become chronic, passing through stages of immune tolerance, immune activation, and inactive infection. Understanding what drives the transitions between these stages is crucial for developing effective therapies.
In this study, using single-cell RNA sequencing and immune receptor profiling, researchers analyzed liver and blood samples from hepatitis B patients at various clinical stages. Their findings show that the transition from immune tolerance to active inflammation is orchestrated by a network of cytotoxic T cells, regulatory macrophages, antibody-producing plasma cells, and neutrophils.
CD8+ T cells were found in multiple functional states, with exhausted T cells dominating during the immune-active phase—highlighting immune dysfunction as a key barrier to viral clearance. At the same time, specialized liver-resident macrophages played a dual role: activating T cells under certain conditions, while also promoting immunosuppression through PD-L1 and IL-10 expression and recruiting regulatory T cells.
The study also revealed that certain liver cells, especially those near the portal vein, are more prone to HBV infection due to high expression of viral entry receptors. These infected cells downregulated immune-activating signals, contributing further to immune evasion. Additionally, increased plasma cell activity and antibody levels, along with neutrophil accumulation and immune-suppressive signaling, were linked to liver inflammation and damage.
By integrating these insights, the research outlines a detailed picture of how immune environments change during HBV infection. This knowledge paves the way for novel treatment approaches that could reinvigorate T cell responses or block key suppressive pathways.
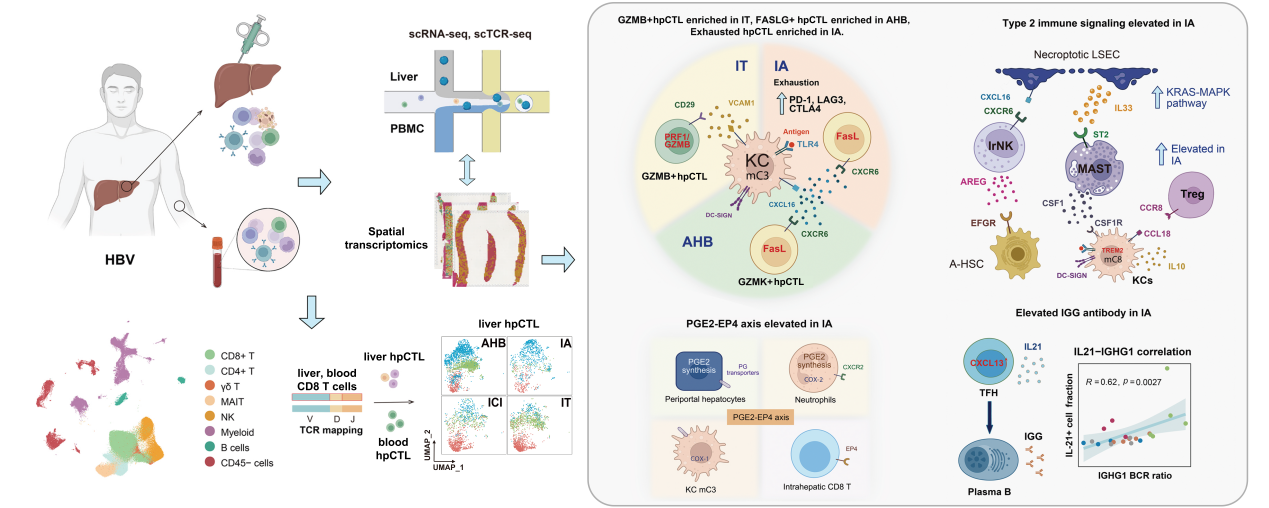
Schematic overview of experimental design and mechanistic insights into cellular immune phenotypes across clinical stages of hepatitis B. (Image by ZHANG Fan)