
A research team led by Prof. GU Hongcang and ZHANG Fan from the Hefei Institutes of Physical Science, Chinese Academy of Sciences, has identified a novel long non-coding RNA (lncRNA)-driven regulatory network that plays a central role in colorectal cancer (CRC) progression and immune response.
The findings, published in Molecular Cancer, highlight potential therapeutic targets that could help overcome treatment resistance in CRC.
Colorectal cancer is marked by significant genetic and epigenetic heterogeneity, which presents a major challenge for current therapies, especially immunotherapy. While immune checkpoint inhibitors have revolutionized cancer treatment, about 85% of CRC patients remain resistant, largely due to molecular complexity.
To better understand these mechanisms, the team conducted an integrative multi-omics analysis—combining transcriptomic, proteomic, and metabolomic data—from CRC tumors and matched normal tissues. Their analysis identified 1,394 differentially expressed lncRNAs, 2,788 mRNAs, 548 proteins, and 91 metabolites.
From this, the researchers built a regulatory network consisting of 22 lncRNAs, 14 mRNAs/proteins, and 9 metabolites. One lncRNA in particular—lncRNA 60967.1—stood out as a key regulator. It was found to be significantly downregulated in both CRC cell lines and patient samples.
Functional experiments showed that restoring lncRNA 60967.1 expression reactivated the tumor suppressor gene PLCD4 and increased levels of all-trans retinoic acid (ATRA). This, in turn, enhanced IFN-γ–induced apoptosis and upregulated IFNGR1, a key receptor subunit for interferon gamma, partially reversing CRC cells’ resistance to immune attack.
In mouse models, overexpression of lncRNA 60967.1 promoted immune cell infiltration and significantly suppressed tumor growth, especially when combined with anti-PD-1 immunotherapy.
This study reveals a new regulatory pathway that affects both tumor growth and immune response in colorectal cancer.
This research was supported by the National Key Technology R&D Program, the National Natural Science Foundation of China, the Seed Fund of the Shanghai Institute of Applied Physics, Chinese Academy of Sciences, the Key R&D Program of Zhejiang Province, and the 2023 Provincial-Ministerial Co-Built Key Projects.
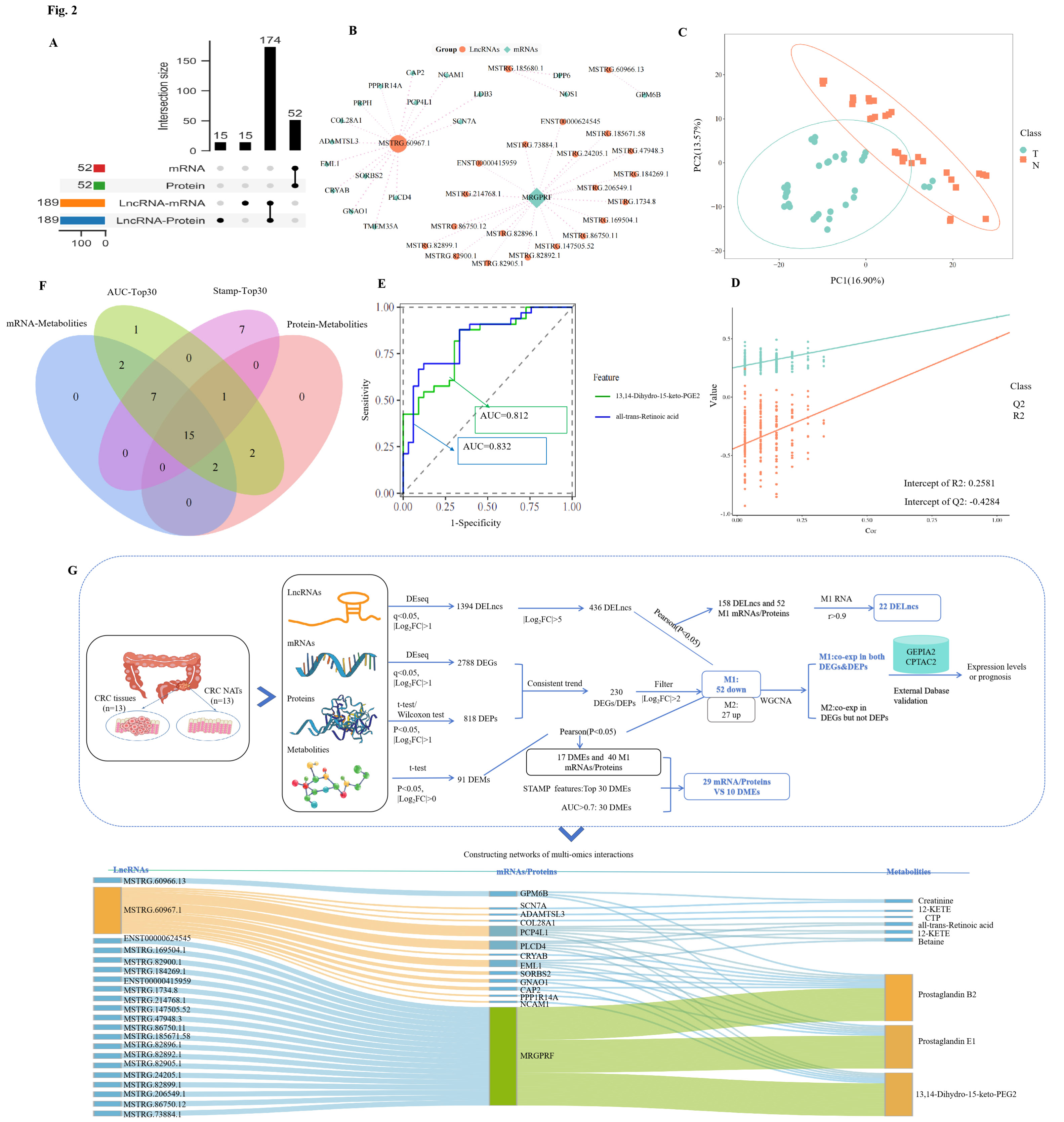
Integrated multi-omics analysis reveals network associations among lncRNAs, mRNAs, proteins, and metabolites. (Image by ZHAO Ningning)
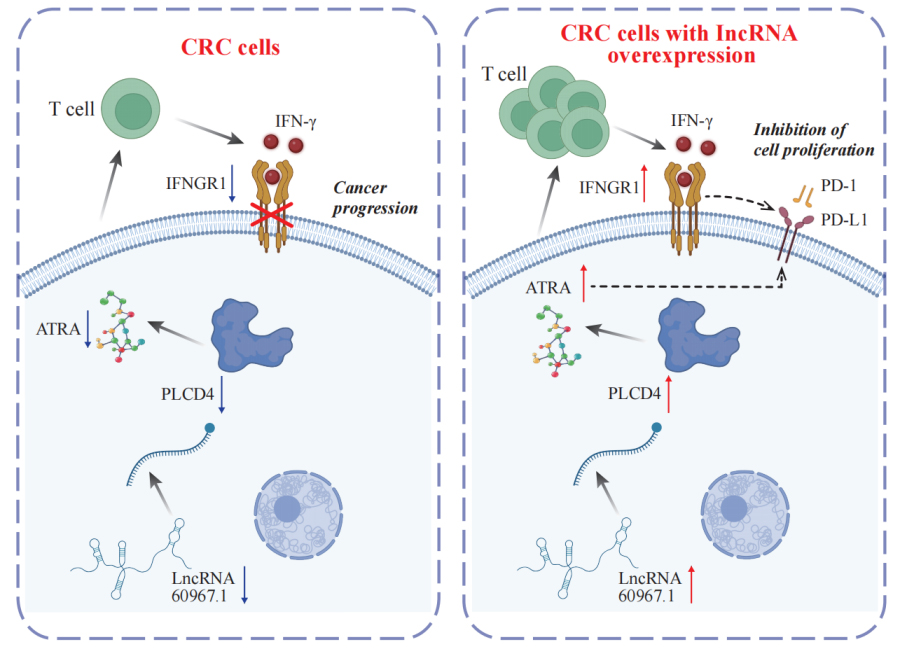
A proposed model suggests that lncRNA 60967.1 plays a regulatory role in modulating the PLCD4/ATRA axis and anti-PD-1 therapy, influencing immune responses and affecting CRC progression. (Image by ZHAO Ningning)