
Recently, a potent and selective enhancer of zeste homolog 2 (EZH2) inhibitor IHMT-EZH2-115 was discovered by a team led by Prof. LIU Qingsong from Hefei Institutes of Physical Science, Chinese Academy of Sciences, for the treatment of B-cell lymphomas.
EZH2 is the enzymatic subunit of polycomb repressive complex 2. As a therapeutic target for the treatment of cancer, it has been extensively studied. Overexpression or mutation of EZH2 has been identified in hematologic malignancies and solid tumors. EPZ6438 (Tazemetostat) is the first selective inhibitor of EZH2 wild-type and mutants approved by the FDA. Despite the clinical success, the diversity of EZH2 inhibitors is still highly demanded for both the preclinical mechanistic and clinical pathological studies.
In this study, starting from EPZ6438 which exhibited anti-B-cell lymphoma efficacies in the preclinical models, researchers obtained a novel EZH2 inhibitor IHMT-EZH2-115 using a focused medicinal chemistry approach guided by computer-aided drug design.
The biochemical assay showed that IHMT-EZH2-115 was highly potent to both EZH2 wild-type and mutants. Meanwhile, it showed high selectivity over a broad range of histone methyltransferases. Furthermore, the inhibitor exhibited excellent antiproliferative activities against cells carrying the heterozygous EZH2 A677G, Y641F, Y641N, and Y641S mutations.
In vivo, IHMT-EZH2-115 exhibited favorable pharmacokinetic characteristics for oral administration and demonstrated dose-dependent antitumor efficacies in two xenograft mouse models of DLBCL cell lines harbouring EZH2 mutations, Pfeiffer (EZH2 A677G) and Karpas-422 (EZH2 Y641N).
These results indicated that IHMT-EZH2-115 might be a potential clinical development candidate for the EZH2 mutant driven tumors.
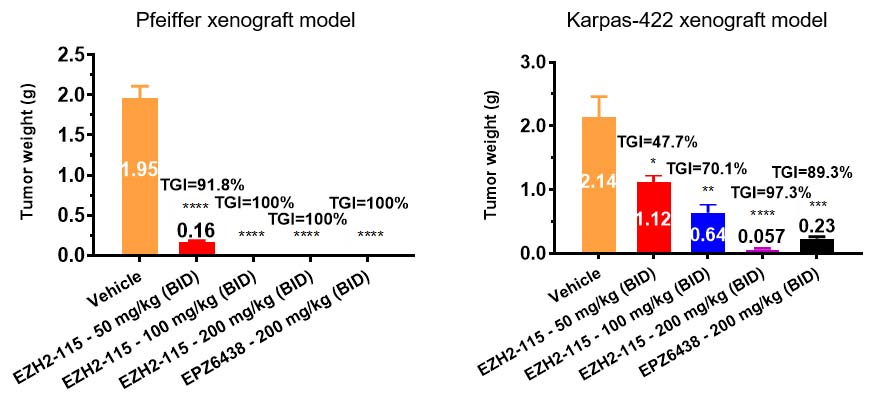
Anti-tumor efficacy evaluation of IHMT-EZH2-115 in xenograft mouse models (Image by ZHOU Bin)