
A research team led by Professor WANG Junfeng at the High Magnetic Field Laboratory, the Hefei Institutes of Physical Science of the Chinese Academy of Sciences, developed a novel 3D bioprinting materials. Their work focuses on creating composite materials using bioactive boron-based glass (BBG) in combination with polycaprolactone (PCL) or sodium alginate (SA), designed for both bone and soft tissue repair.
“By leveraging the bioactivity of BBG and the customizable properties of these materials, we are offering better solutions for tissue repair,” explained Prof. WANG.
The team's findings were recently published in the Materials & Design and the International Journal of Biological Macromolecules.
3D bioprinting is an advanced tissue engineering technique that builds complex tissues using bioactive substances like living cells and scaffolds. It provides personalized tissue repair solutions, reducing immune rejection by using patient-specific cells. Common materials include PCL and PLGA for hard tissue and hydrogels for soft tissue. BBG is effective in bone regeneration by promoting hydroxyapatite formation and has an adjustable degradation rate to suit different repair needs.
The research team developed BBG/PCL composites with varying BBG contents using selective laser sintering (SLS) technology for bone defect repair. After evaluating the materials' pore geometry, porosity, mechanical strength, degradation behavior, and cell compatibility, the team found that the 20% BBG composite was the optimal choice. This material exhibited a porosity of 68.5%, a pore size of 650 microns, and a compressive strength of 0.860 MPa, making it highly effective in supporting bone healing and regeneration.
For soft tissue repair, the researchers turned their attention to BBG-SA bioinks. Using extrusion-based 3D printing technology, they incorporated BBG particles into sodium alginate to create bioinks with high precision and improved gelation properties. Traditional hydrogels often suffer from issues such as low printing accuracy and significant shrinkage during gelation, but the BBG-SA bioinks addressed these problems effectively. The optimal 0.5% BBG-SA hydrogel demonstrated excellent printability, improved structure, and enhanced biocompatibility, encouraging cell adhesion and promoting the expression of genes and proteins associated with soft tissue repair.
“Our work will significantly enhance the potential of bioactive glass in 3D bioprinting materials, providing a crucial research foundation for developing novel bio-based 3D printing materials” said Prof. MA Kun.
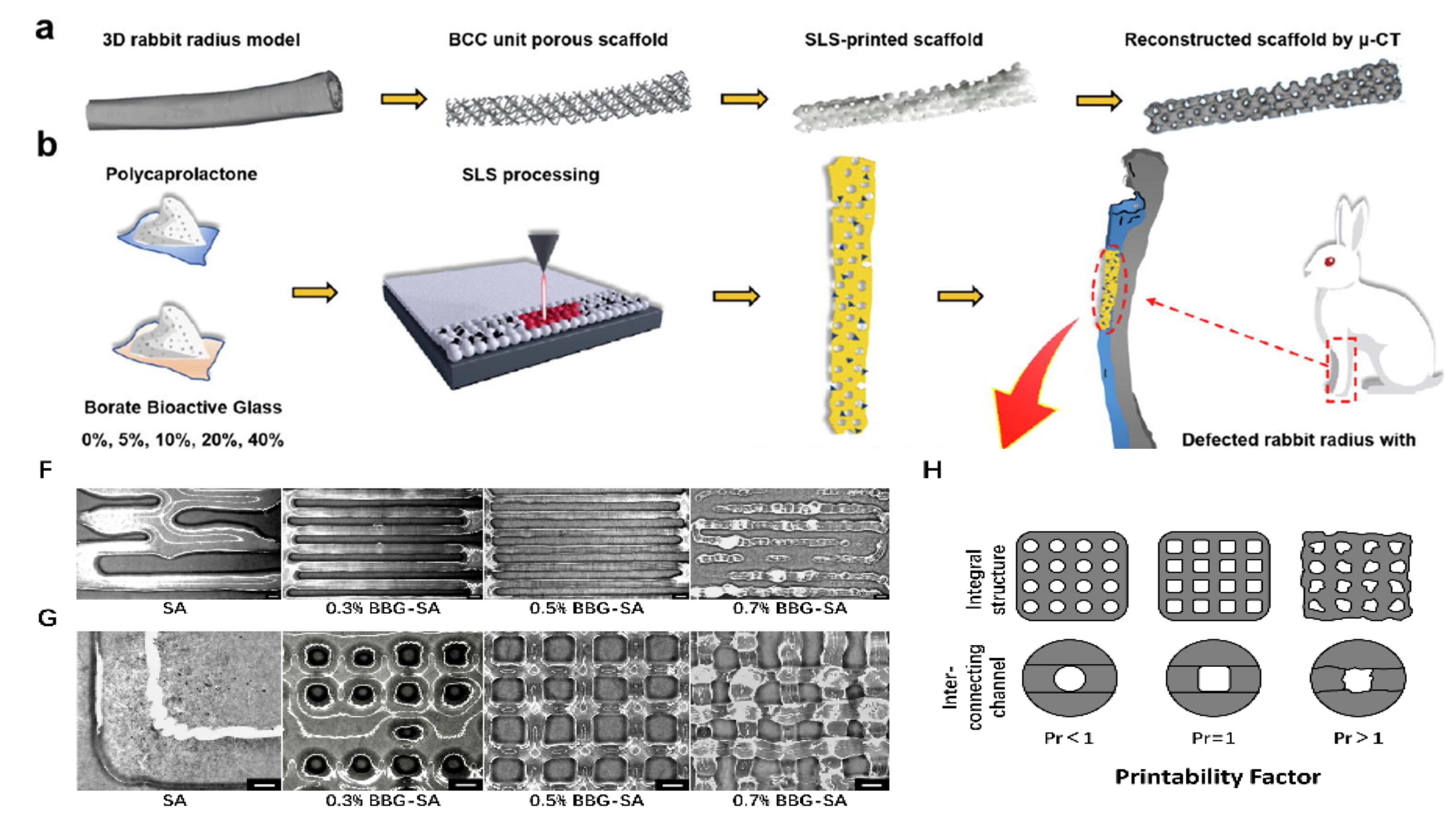
Novel BBG-Based Composite 3D Bioprinting Materials for Tissue Engineering (Image by MA Kun)