
Inhibition of Ubiquitin Specific Peptidase 47 (USP47) was successfully identified as a novel therapeutic target for hematologic malignancies bearing mutant oncoprotein EZH2, according to research conducted by scientists from Hefei Institutes of Physical Science (HFIPS), Chinese Academy of Sciences (CAS). It’s a deubiquitinating enzyme (DUB) that selectively stabilizes mutant EZH2.
The result was published in Leukemia.
Hematologic malignancies are cancers that begin in the bone marrow where blood is produced. Recently, the epigenetic regulator Enhancer of zeste homolog 2(EZH2) has emerged as a crucial target in both diffuse large B-cell lymphoma (DLBCL) and Adult acute myeloid leukemia (AML) due to its high frequency of mutations. However, the secondary mutations and the enzymatic activity independent role of EZH2 caused drug resistance post-long-term treatment of enzymatic inhibitors. Thus, destroying mutant EZH2 protein may be more effective in targeting EZH2 mutant cancers and overriding drug resistance.
In this research, via a chemical genetic screening and combined preclinical models studying, YANG and her colleagues discovered small molecular compounds induced ubiquitin-mediated degradation of mutant EZH2, leading to the death of both AML and DLBCL cells in vitro and in vivo. Using extensive selectivity profiling, they identified USP47, as a novel DUB stabilizing mutant EZH2 and a potential therapeutic target for mutant EZH2-positive hematologic malignancies. Mechanically, USP47 is highly expressed in mutant EZH2-expressing cells, associates with and stabilizes mutant EZH2 over wt EZH2. Inhibition of USP47 spares wt EZH2 in normal cells thus circumventing the adverse side effects in clinical treatment.
The team successfully identified USP10 selectively targeting FLT3-ITD and kinase inhibitor-resistant FLT3 mutants over wt FLT3 in AML, and JOSD1 inhibitors that selectively targeting JAK2-V617F in AML.
According to YANG Jing, the first author of the paper, therapeutic targeting of mutant EZH2 by promoting its degradation via DUB inhibition as opposed to inhibition of its catalytic activity is an innovative approach that may be beneficial for overcoming resistance to current EZH2 inhibitors.
"Our findings provide potential benefits for both avoiding the adverse side effects associated with EZH2 inhibition in clinical using and also for overcoming the technical limitations associated with EZH2 inhibition that reduce clinical efficacy,” explained WEISBERG Ellen, the co-first author from Dana-Farber Cancer Institute (DFCI), Harvard Medical School.
The research was supported by National Natural Science Foundation of China, Natural Science Foundation of Anhui Province, National Institutes of Health (NIH) Foundation R01, and et al.
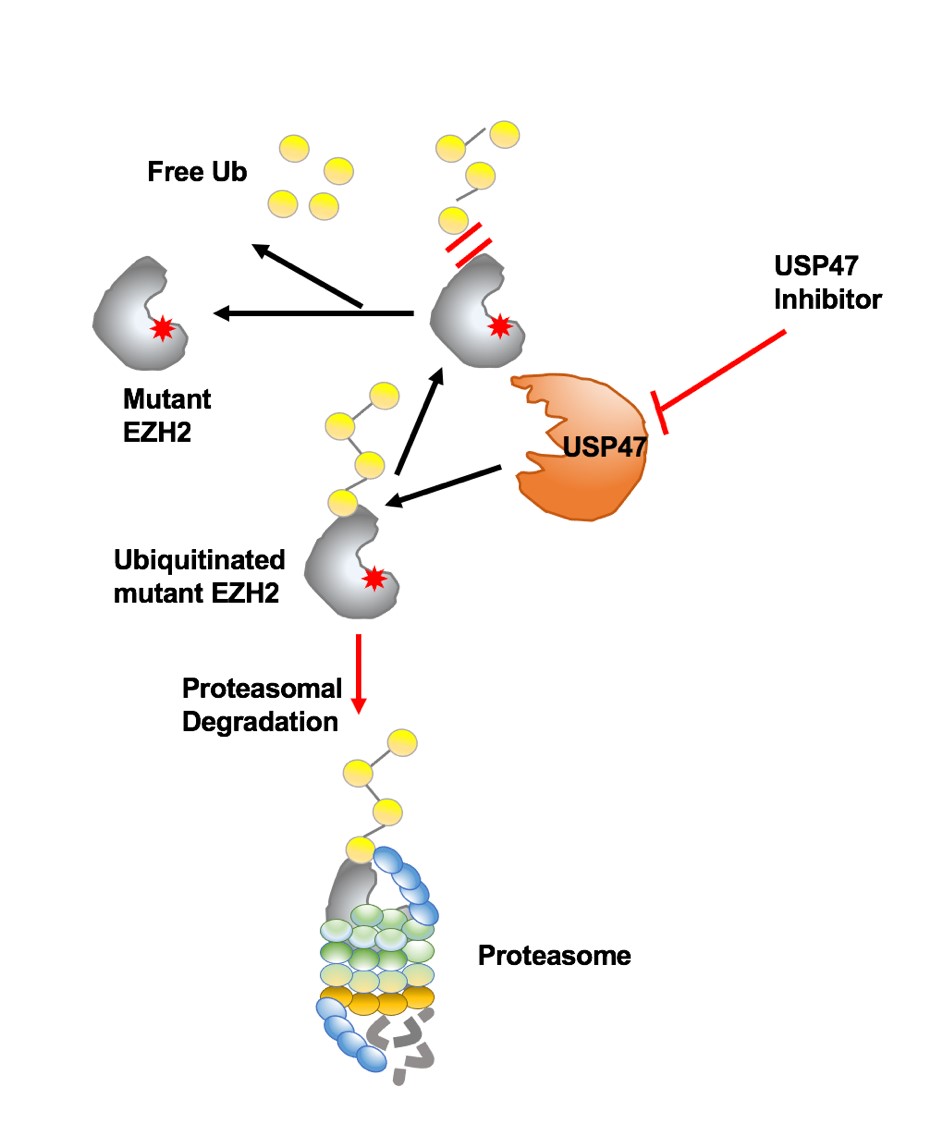
Figure 1. Proteasome-mediated degradation of mutant EZH2 proteins. USP47 inhibitor treatment targeted oncogenic EZH2 protein degradation through activation of proteasomal-mediated degradation.(Image by YANG Jing)