
Urea (CO(NH2)2) has been applied both in agricultural and pharmaceutical field. The Bosch-Meiser process widely used now results in high energy consumption and CO2 emission. Therefore, it is imperative to explore energy-saving and economical routes for urea synthesis under mild conditions.
However, the electrosynthesis of urea with CO2 and NO3- under ambient conditions,which is an efficient way, is still far away from application. This is because the key step needs an efficient electrocatalyst enabling adsorption and activation of NO3- and CO2 to accomplish the C-N coupling.
Researchers from Hefei Institutes of Physical Science of Chinese Academy of Sciences has now developed a liquid-phase laser irradiation route to fabricate symbiotic carbon encapsulated amorphous iron (Fe(a)@C) and iron oxide nanoparticles (Fe3O4 NPs) on carbon nanotubes (denoted as Fe(a)@C-Fe3O4/CNTs).
The as-fabricated Fe(a)@C-Fe3O4/CNTs contained two Fe-based active components, namely, Fe@C NPs with the particle sizes of 10~20 nm and Fe3O4 NPs with the particle sizes of 1~5 nm.
The presence of two different structural units in Fe(a)@C-Fe3O4/CNTs made it possible to synergistically electrocatalytic activate CO2 and NO3- to realize the C-N coupling for urea synthesis.
As expected, Fe(a)@C-Fe3O4/CNTs exhibited superior activity toward the electrocatalytic coupling of CO2 and NO3- for urea synthesis, affording a urea yield of 1341.3±112.6 μg h-1 mgcat-1 and a faradic efficiency (FE) of 16.5±6.1% at -0.65 V (vs. RHE) in 0.1 M KNO3 electrolyte.
Both experimental and theoretical results unveiled that Fe(a)@C was mainly responsible for the electrocatalytic reduction of NO3- to form *NH2 intermediates, while Fe3O4 was more beneficial for the electrocatalytic reduction of CO2 to form *CO intermediates.
The synergistically catalytic effect contributes the excellent electrocatalytic performance of urea synthesis at ambient conditions.
This work was financially supported by the Natural Science Foundation of China.
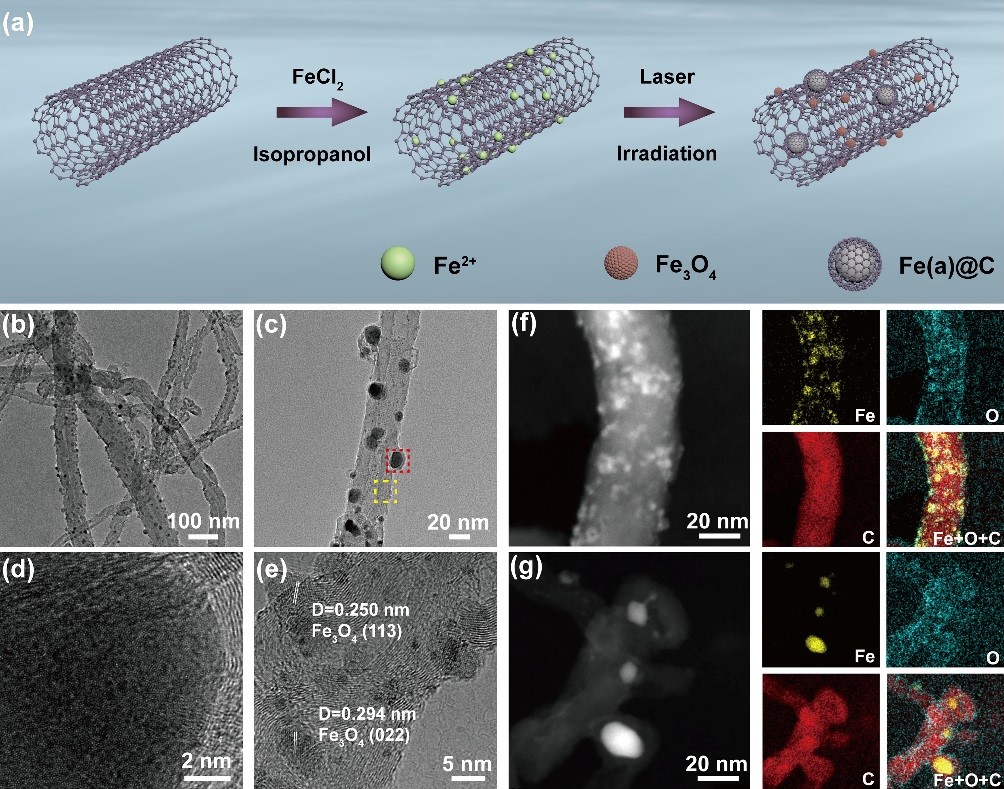
(a) Schematic illustration of the synthetic process of Fe(a)@C-Fe3O4/CNTs. (b) Low- and (c) high-magnification TEM images of Fe(a)@C-Fe3O4/CNTs. HRTEM images of Fe(a)@C (d) (from the red dashed box in c) and Fe3O4 NPs (e) (from the yellow dashed box in c). (f), (g) HAADF-STEM images and corresponding elemental mapping images of Fe3O4 and Fe(a)@C. (Image by GENG Jing)