
Recently, the research team led by Professor WANG Junfeng from the High Magnetic Field Laboratory of Hefei Institutes of Physical Science (HFIPS), Chinese Academy of Sciences (CAS), successfully developed a novel heat-sensitive ferritin mutant based on an in-depth understanding of the human ferritin molecular structure. They achieved the easy and efficient loading of the chemotherapy drug doxorubicin.
The findings were published in International Journal of Biological Macromolecules.
Human ferritin forms a stable nanocage by itself. Human ferritin's nanocage shape and natural targeting capabilities have been exploited for loading medicinal compounds, imaging agents, nucleic acids, and more for decades. Different diseases have been diagnosed and treated using it. However, current drug loading techniques for ferritin are complex and require strict reaction conditions including high temperatures and strong acids/bases. Ferritin-based nanomedicine has limited drug loading and protein recovery rates, limiting its clinical use.
In this study, through an in-depth analysis of the surface structure of ferritin, which included eight hydrophilic triphase channels and six hydrophobic tetraphase channels, the research team successfully predicted the correlation between the four-phase channel structure domain of ferritin and its heat sensitivity.
Based on this discovery, they developed Ecut-HFn, a unique and highly heat-sensitive ferritin mutant. They achieved one-step drug loading at low temperatures (45°C) with the help of copper ions.
"There are various advantages to using this new ferritin-based medication loading strategy," said MA Kun, member of the team.
On one hand, the entire encapsulation process had no requirement for acid/base reagents or high-temperature treatment, making it eco-friendly. It was suitable for loading biologically active compounds that were sensitive to acid/base and high-temperature conditions.
On the other hand, the shell-like structure of ferritin remained intact throughout the process, with almost no protein disassembly involved. This enabled high protein recovery rates and preserved the natural tumor-targeting ability of ferritin.
Also, there was a significant improvement in drug loading efficiency.
This research not only provided a comprehensive understanding of the relationship between the four-phase channel structure domain of ferritin and its heat sensitivity but also offered valuable insights into expanding the development of novel drug loading methods based on the structural modification of human ferritin, according to the team.
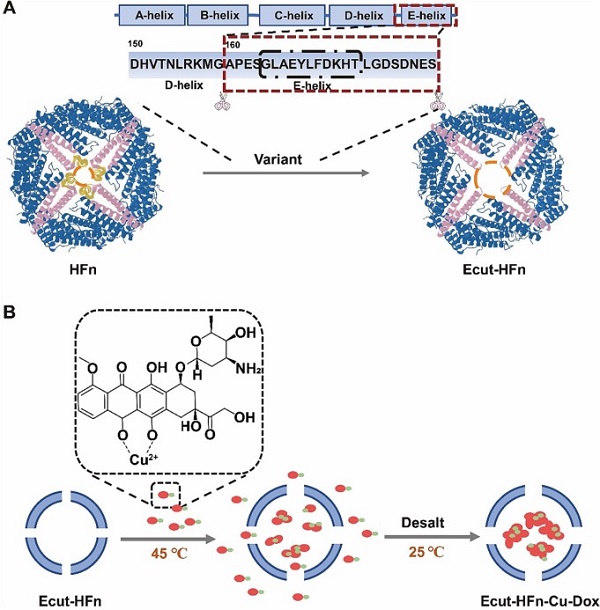
Schematic diagram of Cu2+-assisted Ecut-HFn thermoresponsive drug loading. (Image by MA Kun)