
A research team led by Prof. ZHANG Haimin from the Institute of Solid State Physics, Hefei Institutes of Physical Science (HFIPS) of Chinese Academy of Sciences (CAS) has conducted a systematic study on the regulation mechanism of heterostructure bimetallic phosphide electrocatalysts for enhanced electrochemical nitrate reduction reaction performance.
"The catalyst exhibits varying ammonia synthesis performance in different reactors," said Dr. Jinmeng, "so we has tried three different electrolyzers to determine how the performance of the electrocatalysts could be improved."
"The configuration of the electrolyzer will greatly affect the local reaction environment near the electrode, and then affect the catalytic performance", said Dr. Jinmeng, "so we have chosen three different electrolyzers to study the regulate mechanism of the electrocatalysts performance."
The result was published in Nano Research.
The nitrate anion (NO3- ) is significant pollutant in industrial wastewater and agriculture production. Electrocatalytic nitrate reduction (NO3RR) is an effective path to solving environmental problems and the preparation of green ammonia (NH3). However, the NO3RR process is complex, involving multiple electrons and protons transfer, and suffers from low Faraday efficiency due to competition of hydrogen evolution reactions. Electrochemical NO3RR reactor is also critically for achieving high-efficiency conversion of NO3- to NH3.
In this study, researchers synthesized a series of bimetallic copper-nickel phosphide electrocatalysts on commercial carbon paper (CP) via a facile vapor-phase hydrothermal method. The electrocatalytic performance of these catalysts for NO3RR was first evaluated in an H-type electrolytic cell. The results showed that the Cu3P-Ni2P/CP-x could establish rich heterointerfaces which boosted the electron transfer, and improved the NO3RR efficiency.
To further understand the the kinetics of electrocatalytic NO3RR, researchers employed a rotating disk electrode (RDE) setup to explore performance variations.
To further understand the NO3RR kinetics difference of electrocatalysts, researchers employed a rotating disk electrode (RDE) setup to test the corresponding catalytic kinetics parameters.
In addition, they assembled the catalyst into the membrane-electrode-assemblies (MEA) electrolyzer, exhibiting the high-efficient activity and durability for NO3RR at industrial current densities. In-situ spectroscopy characterization, combined with theoretical calculations, revealed that the presence of heterointerfaces effectively regulated reactant adsorption, and the reaction mechanism followed a successive hydrodeoxygenation pathway.
These findings contribute to a better understanding of the electrocatalytic NO3RR process and pave the way for the development of efficient and durable catalysts for sustainable ammonia synthesis.
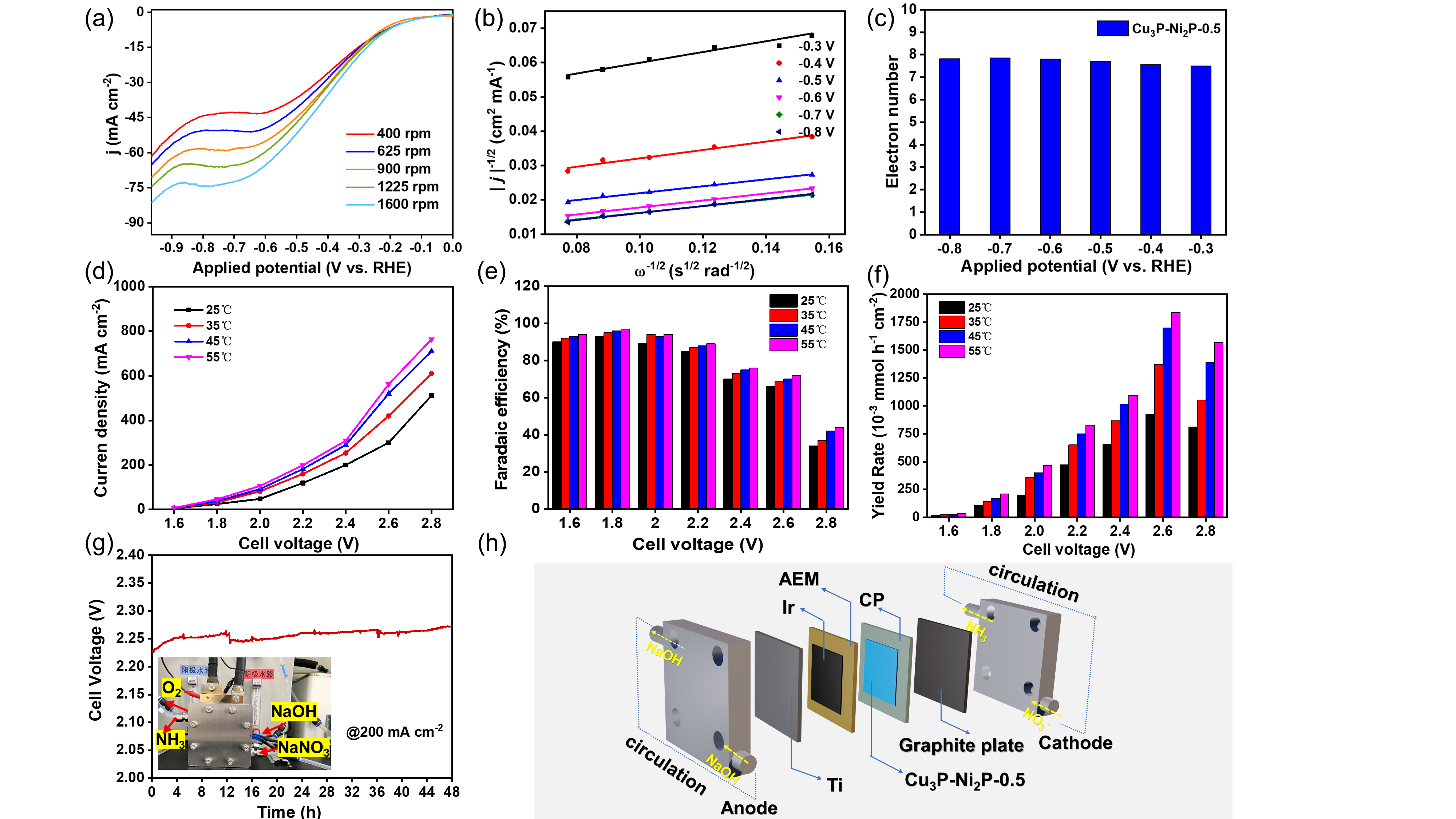
Fig. 1 The electrocatalytic NO3RR kinetic test and its scale-up for practical applications. (Image by JIN Meng)
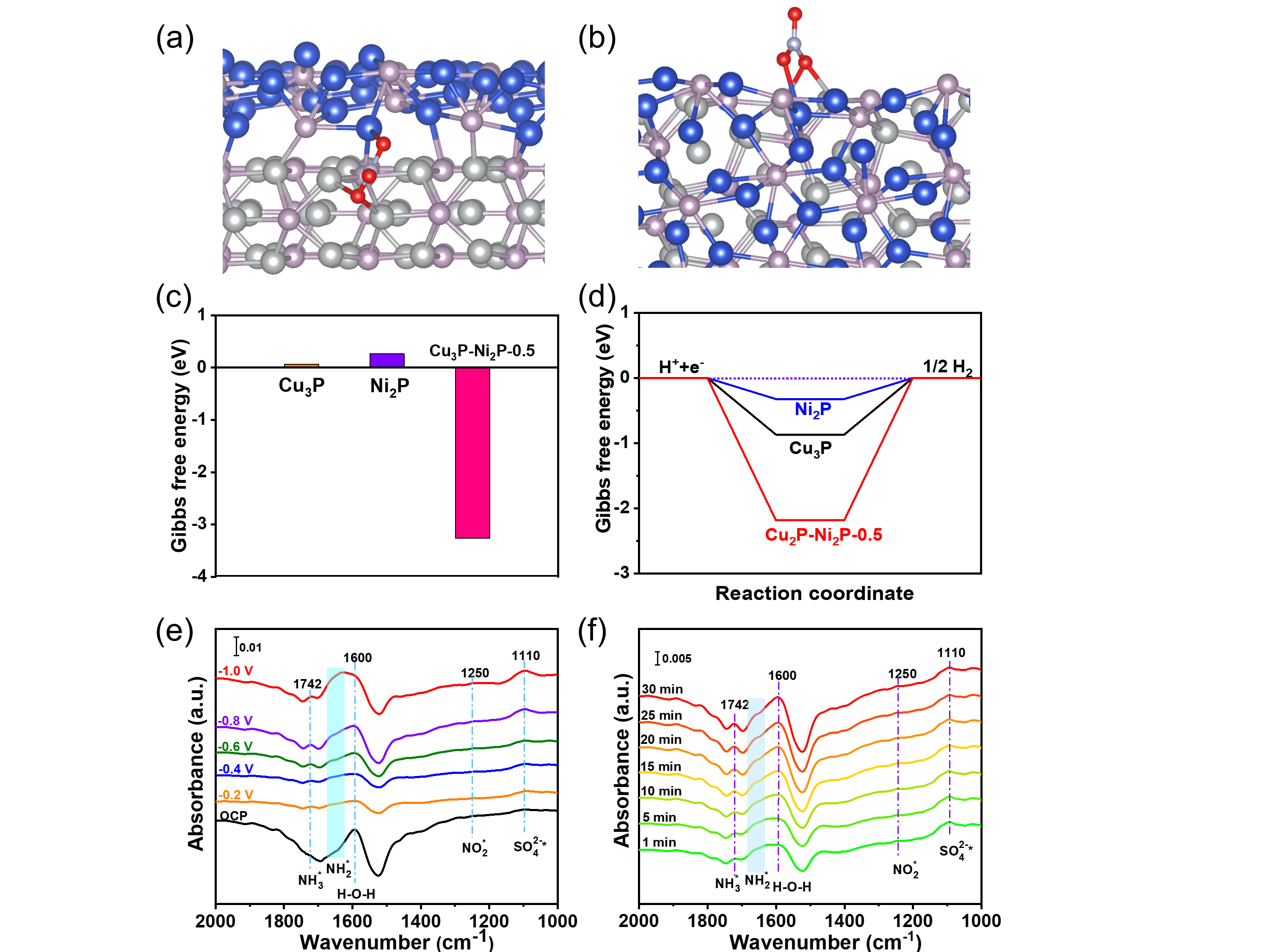
Fig. 2 Theoretical calculations and In-situ spectroscopy characterization toward NO3RR. (Image by JIN Meng)