
A research team led by Professor ZHAO Guoping at the Hefei Institutes of Physical Science of the Chinese Academy of Sciences, has unveiled a crucial mechanism that helps regulate DNA damage repair, with important implications for improving cancer treatment outcomes.
The result was published in Cell Death & Differentiation.
The efficacy of radiotherapy is largely limited by the DNA damage repair capacity of tumor cells. When ionizing radiation induces DNA double-strand breaks — the primary lethal damage — tumor cells often exhibit abnormal overexpression of DNA repair proteins, establishing a robust damage response system that drives clinical radioresistance. To address this challenge, the team deciphered the regulatory network of epigenetic modifications in DNA damage repair.
The study found that ZNF451, a zinc finger protein, is significantly overexpressed in breast cancer, lung cancer, and other malignancies, correlating with poor patient prognosis. Mechanistic studies demonstrated that upon radiation-induced DNA damage, ZNF451 rapidly accumulates at damage sites and specifically catalyzes SUMO2 modification of RNF168. This post-translational modification stabilizes RNF168, enhancing its recruitment to damage sites, which amplifies downstream ubiquitination of histone H2A/H2AX and ultimately promotes DNA repair.
The team further uncovered a dynamic regulatory network between ZNF451 and the canonical repair factor RNF8. These two proteins modulate RNF168 activity through competitive binding and cooperative interactions. Specifically, ZNF451 and RNF8 mutually inhibit each other' s recruitment of RNF168, yet simultaneous depletion of both severely impairs RNF168 accumulation at damage sites. Quantitative analysis revealed that ZNF451 and RNF8 dose-dependently fine-tune the interaction strength between RNF168 and its substrate H2AX.
This study not only elucidates a novel mechanism by which SUMOylation regulates DNA damage repair but also proposes an innovative "dynamic equilibrium regulation" model, offering theoretical advancements in understanding DNA repair mechanisms.
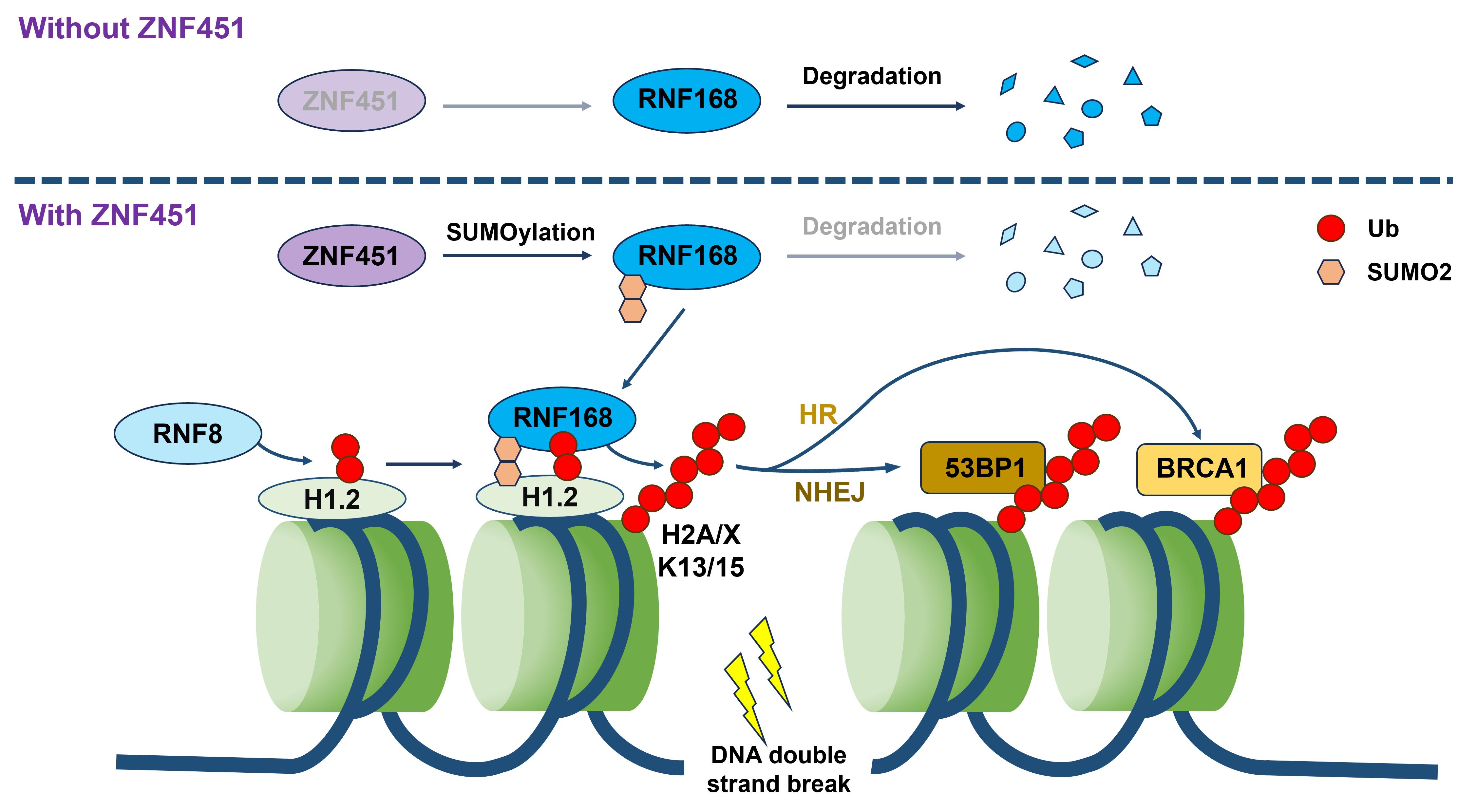
Schematic illustration of ZNF451 regulating DNA damage repair mechanism (Image by ZHAO Guoping)