
With the help of the Nuclear Magnetic Resonance (NMR) spectrometer, a research group led by Prof. WANG Hui from High Magnetic Field Laboratory, Hefei Institutes of Physical Science of Chinese Academy of Sciences, successfully prepared a carbon-covered hollow cuprous oxide high-efficiency catalyst by using solvent autocarbonylation reduction strategy, which offered a new solution for the electrocatalytic CO2 Reduction Reaction (CO2RR) during the preparation of multicarbon (C2+) products.
The research results were published in Advanced Functional Materials.
Excessive carbon dioxide emissions are a global concern. Converting CO2 into chemicals and fuels through CO2RR not only helps the environment but also supports the "dual-carbon" goal. While progress has been made in producing single carbon (C1) products like carbon monoxide and formic acid from CO2RR, attention is shifting to C2+ products due to their higher energy density and economic value. However, current CO2RR efficiency in producing C2+ products is low, creating a need for catalysts that can enhance efficiency and selectivity.
In this study, the team developed a specialized nanoreactor called nitrogen-doped carbon shell-protected hollow cuprous oxide (H-Cu2O@C/N) using a solvent autocarbonation reduction strategy.
This nanoreactor increase helps increase the amount of important intermediates (*CO) on the catalyst surface, which speeds up the production of C2+ products through a chemical reaction.
When tested in a membrane electrode assembly (MEA) electrolyzer, the H-Cu2O@C/N nanoreactor achieved impressive results, with a 75.9% efficiency in producing C2+ products and a high current density of 248.8 mA·cm-2. This proves effectiveness of the catalysts in converting CO2RR.
In order to further understand this process, the research team conducted detailed studies. These results confirmed that the C/N inclusions prepared by solvent autocarbon reduction strategy can effectively protect the Cu+ active species and ensure their catalytic stability.
This work provides an efficient and feasible way to optimise the catalyst structure for highly selective CO2RR preparation of C2+ products.
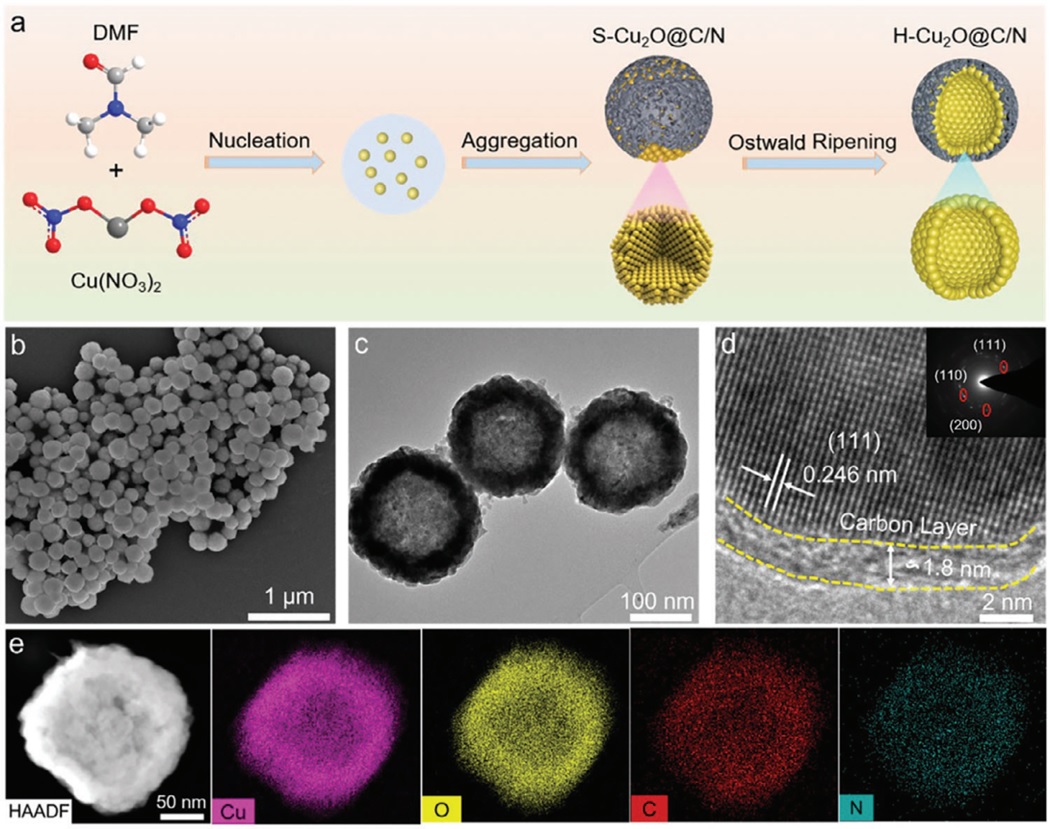
Figure 1. Synthesis schematic and characterization of H–Cu2O@C/N. a) Schematic of the synthetic process for H–Cu2O@C/N. b) SEM image, c) TEM
image, d) HRTEM image, and the corresponding selective area electron diffraction (SAED) pattern (inset) of H–Cu2O@C/N.